OMLONTI Ophthalmic solution Ref.[50996] Active ingredients: Omidenepag
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
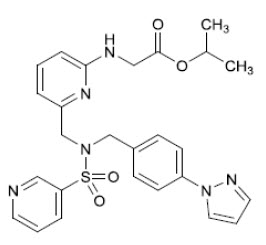
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, contains the prodrug form of the active omidenepag, a relatively selective prostaglandin EP2 receptor agonist with ocular hypotensive activity. Omidenepag isopropyl is a white to light brown crystal or crystalline powder and practically insoluble in water. Omidenepag isopropyl’s chemical name is Glycine, N-[6-[[[[4-(1H-pyrazol-1-yl)phenyl]methyl](3-pyridinylsulfonyl)amino]methyl]-2-pyridinyl]-, 1-methylethyl ester and has the following structure:
Structural Formula:
Formula of the free base: C26H28N6O4S.
Molecular weight: 520.61
Omlonti appears as a clear, colorless solution. It is supplied as a sterile, isotonic, buffered aqueous solution of omidenepag isopropyl with a target pH of 5.8 and an osmolality of approximately 285 mOsmol/kg.
Each mL of Omlonti contains:
Active: 0.02 mg of omidenepag isopropyl.
Preservative: 0.005% benzalkonium chloride.
Inactive ingredients: glycerin, polyoxyl 35 castor oil, sodium citrate, citric acid monohydrate, edetate disodium, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection.
| Dosage Forms and Strengths |
|---|
|
Omidenepag isopropyl ophthalmic solution: 0.002% (0.02 mg/mL). |
| How Supplied |
|---|
|
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is supplied as a 2.5 mL sterile solution in 5 mL white low density polyethylene bottles with linear low density polyethylene dropper tips, high density polyethylene screw caps and tamper-evident low density polyethylene overcaps. NDC 65086-002-05 Manufactured for: Santen Inc. Manufactured by: Woodstock Sterile Solutions, Inc. Distributed by: Santen Inc., 6401 Hollis Street, Suite 125, Emeryville, CA 94608, United States |
Drugs
| Drug | Countries | |
|---|---|---|
| OMLONTI | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.