OMNIPAQUE Solution Ref.[9483] Active ingredients: Iohexol
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2019 Publisher: GE Healthcare, 300 Great South Road, PO Box 17122, Greenlane, Auckland 1130 Ph (09) 523-5896 Fax (09) 522-7342
Therapeutic indications
This medicinal product is for diagnostic use only.
Intravascular
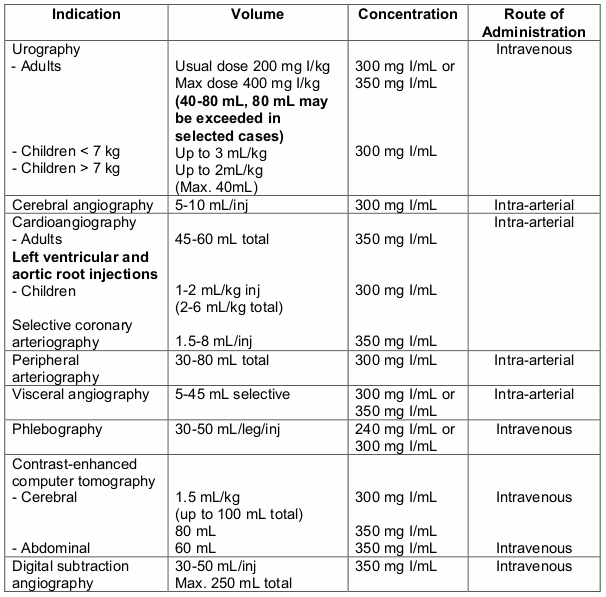
OMNIPAQUE is indicated in adults for angiography, excretory urographyand CT-enhancement. In children, OMNIPAQUE is indicated for angiography and urography.
Intrathecal
OMNIPAQUE is indicated for lumbar, thoracic, cervical and total columnar myelography and computed tomography of the CNS in adults and children.
Oral/Body cavities
OMNIPAQUE is indicated in adults in arthrography, endoscopic retrograde pancreatography (ERP), endoscopic retrograde cholangiopancreatography (ERCP), herniography, hysterosalpingography, and in adults, children and prematurte babies for studies of the gastrointestinal tract.
Posology and method of administration
Administration of contrast media should be performed by qualified personnel familiar with the procedure, and an appropriate technique should be utilised.
The dosage varies depending on the type of examination, age, weight, cardiac output and general condition of the patient and the technique used. Adequate hydration should be assured before and after administration as for other contrast media.
As in all diagnostic procedures, the lowest dose of OMNIPAQUE necessary to obtain adequate visualisation should be used. Most procedures do not require use of either the maximum volume or the highest concentration of OMNIPAQUE. The combination of volume and concentration of OMNIPAQUE to be used should be carefully individualised accounting for factors such as age, body weight, size of the vessel and the rate of blood flow within the vessel. Other factors such as anticipated pathology, degree and extent of opacification required, structure(s) or area to be examined, disease process affecting the patient, and equipment and technique to be employed should be considered.
Patients will tolerate a contrast medium better if the contrast medium is warmed to body temperature, which lowers the viscosity.
OMNIPAQUE should be inspected visually for particulate matter, discolouration and the integrity of the container prior to administration. OMNIPAQUE should only be used if clear and within the normal colourless to pale yellow range. Do not use if particulate matter or discolouration are present.
The following dosages may serve as a guide.
Guidelines for Intravascular use
Guidelines for Intrathecal use
To minimise possible adverse reactions a total dose of 3 g iodine should not be exceeded. As in all diagnostic procedures, the minimum concentration and volume to produce adequate visualisation should be used.
To avoid excessive mixing with CSF and consequent dilution of contrast, as well as premature dispersion upwards, injection must be made slowly. Depending on the estimated volume of OMNIPAQUE which may be required for the procedure, a small amount of CSF may be removed to minimise the distension of the subarachnoid spaces.
The needle may be removed immediately following injection since it is not necessary to remove OMNIPAQUE after injection into the subarachnoid space. An interval of 48 hours should be allowed before repeat examination.
Direct intracisternal or ventricular administration for standard radiography (without computerized tomographic enhancement) is not recommended.
Adults
The usual recommended total doses of OMNIPAQUE are:
| Procedure | Concentration (mg I/mL) | Volume (mL) |
|---|---|---|
| Lumbar myelography | 180 | 8-17 |
| Thoracic myelography | 240 | 7-10 |
| Cervical or total columnar myelography (via lumbar puncture) | 300 | 6-10 |
| Cervical myelography (via lateral cervical injection) | 240 or | 8-10 |
| 300 | 5-10 |
Infants and Children
OMNIPAQUE 180 mg I/mL is recommended for the examination of the lumbar, thoracic and cervical regions in children by lumbar injection and is slightly hypertonic to CSF. The usual recommended total doses for myelography (by lumbar injection) are given below, depending largely on patient age.
| Age | Concentration (mg I/mL) | Volume (mL) |
|---|---|---|
| 3-36 months | 180 | 4-8 |
| 3-7 years | 180 | 5-10 |
| 7-13 years | 180 | 5-12 |
| 13-18 years | 180 | 6-15 |
Avoid rapid dispersion of the medium. (To avoid excessive mixing with cerebrospinal fluid (CSF) and consequent dilution of iohexol solution, injection should be made slowly over 1 to 2 minutes.)
If repeat examinations are desired, a suitable interval of time between administrations is needed to allow for normal clearance of the drug from the body. An interval of at least 48 hours should be allowed before repeat examination; however, 5 to 7 days is recommended whenever possible.
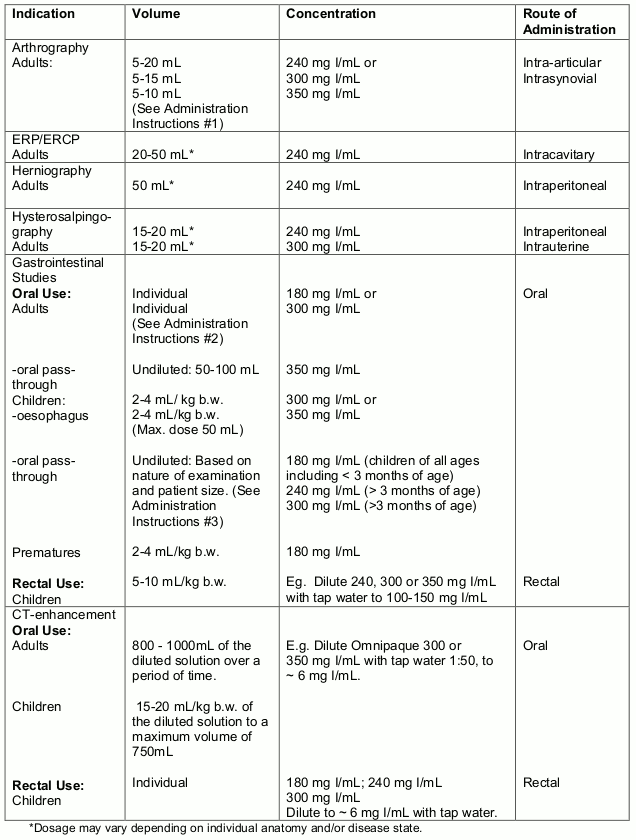
Guidelines for Body Cavities/Oral Use
Administration Instructions for Body Cavities/Oral Use
Arthrography
The amount of Omnipaque injected is dependent on the size of the joint to be examined and the technique employed. Lower volumes of contrast medium are usually injected for knee and shoulder arthrography when double-contrast examinations using 15 mL to 100 mL of air are performed.
The following concentrations and volumes are recommended for normal adult knee, shoulder and temporomandibular joints but should serve as guidelines since joints may require more or less contrast medium for optimal visualisation.
Knee:
Omnipaque 240 5 mL to 15 mL.
Omnipaque 300 5 mL to 15 mL.
Omnipaque 350 5 mL to 10 mL.
Shoulder:
Omnipaque 300 10 mL.
Omnipaque 240 3 mL.
Temporomandibular:
Omnipaque 300 0.5 mL to 1.0 mL.
Lower volumes recommended for double-contrast examinations; higher volumes recommended for single contrast examinations. Passive or active manipulation is used to disperse the medium throughout the joint space.
Oral Use
Adults:
The recommended dosage of undiluted Omnipaque 350 at a concentration of 350 mg I/mL for oral pass-through examination of the gastrointestinal tract in adults is 50 mL to 100 mL depending on the nature of the examination and the size of the patient.
OMNIPAQUE diluted to concentrations from 6 mg I/mL to 9 mg I/mL and administered orally in conjunction with OMNIPAQUE 300 at a concentration of 300 mg I/mL administered intravenously is indicated in adults for use in contrast enhanced computed tomography of the abdomen. Dilute oral plus intravenous OMNIPAQUE may be useful when unenhanced imaging does not provide sufficient delineation between normal loops of the bowel and adjacent organs or areas of suspected pathology.
The recommended oral dosage of OMNIPAQUE dilute to concentrations of 6 mg I/mL to 9 mg I/mL for contrast enhanced computed tomography of the abdomen in adults is 500 mL to 1000 mL. Smaller administered volumes are needed as the concentration of the final solution is increased. In conjunction with dilute oral administration, the recommended dosage of OMNIPAQUE 300 administered intravenously is 100 mL to 150 mL. The oral dose is administered about 20 to 40 minutes prior to the intravenous dose and image acquisition.
Children:
The dosage of undiluted OMNIPAQUE 300 at a concentration of 300 mg I/mL, OMNIPAQUE 240 at a concentration of 240 mg I/mL or OMNIPAQUE 180 at a concentration of 180 mg I/mL for oral pass-through examination of the gastrointestinal tract is dependent on the nature of the examination and the size of the patient. Based on clinical experience, it is recommended that OMNIPAQUE 180 be used in children less than 3 months of age. OMNIPAQUE 180, OMNIPAQUE 240 or OMNIPAQUE 300 may be used in children 3 months of age and older. The recommended does for oral use in children is 2-4mL/kg. The estimated total volumes based on this dosage are:
| Age | Volume of OMNIPAQUE |
|---|---|
| Less than 3 months | 5-30 mL |
| Three months to 3 years | Up to 60 mL |
| Four years to 10 years | Up to 80 mL |
| Greater than 10 years | Up to 100 mL |
When given rectally, larger volumes may be used.
OMNIPAQUE diluted to concentrations from 9-21 mg I/mL administered orally in conjunction with OMNIPAQUE 240 at a concentration of 240 mg I/mL or OMNIPAQUE 300 at a concentration of or 300 mg I/mL administered intravenously is indicated in children for use in contrast enhanced computed tomography of the abdomen. The recommended dose is 15-20 mL/kg up to a maximum volume of 750 mL. Smaller administered volumes are needed as the concentration of the final solution is increased. The total oral dose in grams of iodine should generally not exceed 5 g for children less than 3 years of age and 10 g for children from 3 to 18 years of age. The oral dosage may be given all at once or over a period of 30 to 45 minutes if there is difficulty consuming the required volume.
In conjunction with dilute oral administration the recommended dosage of OMNIPAQUE 240 and OMNIPAQUE 300 is 2.0 mL/kg when administered intravenously with a range of 1.0 mL/kg to 2.0 mL/kg. Dosage for infants and children should be administered in proportion to age and body weight. The total intravenously administered dose should not exceed 3 mL/kg. The oral dose is administered about 30 to 60 minutes prior to the intravenous dose and image acquisition.
Overdose
Preclinical data indicate a high safety margin for OMNIPAQUE and no fixed upper dose level has been established for routine intravascular use. Symptomatic overdosing is unlikely in patients with normal renal function unless the patient has received an excess of 2000 mg I/kg body-weight over a limited period of time.
The duration of the procedure is important for the renal tolerability of high doses of contrast media (t1⁄2 ~ 2 hours). Accidental overdosing is most likely following complex angiographic procedures in children, particularly when multiple injections of contrast medium with high- concentration are given.
Clinical consequences of overdosage with OMNIPAQUE have not been reported. However, based on experience with other non-ionic myelographic media, physicians should be alert to a potential increase in the frequency and severity of CNS-mediated reactions. Even use of the recommended dose can produce effects tantamount to overdosage if incorrect management of the patient during or immediately following the procedure permits inadvertent early intracranial entry of a large portion of the medium.
The maximum recommended dose of OMNIPAQUE by intrathecal administration is 3 g of iodine.
In cases of overdose, any resulting water or electrolyte imbalance must be corrected. Renal function should be monitored for the next 3 days. If needed, haemodialysis may be used for clearance of excessive contrast medium. There is no specific antidote.
In case of overdose, immediately contact the Poisons Information Centre for advice, in New Zealand, call 0800 764 766.
Shelf life
Shelf life
Glass or polypropylene bottles (10–150 ml): 36 months.
Polypropylene bottles (200–500 ml): 24 months.
Special precautions for storage
OMNIPAQUE should be stored at 2-30oC protected from light and secondary X-rays. The product in glass vials and bottles may be stored at 37°C for up to 3 months prior to use.
The product in 50 ml polypropylene bottles may be stored at 37°C for up to 1 month prior to use. The product in 10 ml and 20 ml polypropylene bottles may be stored at 37°C for up to 1 week prior to use.
Nature and contents of container
180 mg I/ml:
10 ml: Packs of 10 glass or polypropylene bottles.
240 mg I/ml:
20 ml: Packs of 6 or 25 glass bottles and 10 polypropylene bottles.
50 ml: Packs of 10 glass or polypropylene bottles.
500 ml: Packs of 6 bottles.
300 mg I/ml:
10 ml: Packs of 10 glass or polypropylene bottles.
20 ml: Packs of 6 or 25 glass bottles and 10 polypropylene bottles.
50 ml: Packs of 10 glass or polypropylene bottles.
75 ml: Packs of 10 glass or polypropylene bottles.
100 ml: Packs of 10 glass or polypropylene bottles.
150 ml: Packs of 10 polypropylene bottles.
200 ml: Packs of 10 polypropylene bottles.
500 ml: Packs of 6 glass or polypropylene bottles.
350 mg I/ml:
20 ml: Packs of 6 or 25 glass bottles and 10 polypropylene bottles.
50 ml: Packs of 10 glass or polypropylene bottles.
75 ml: Packs of 10 glass or polypropylene bottles.
100 ml: Packs of 10 glass or polypropylene bottles.
150 ml: Packs of 10 polypropylene bottles.
200 ml: Packs of 6 glass or 6 and 10 polypropylene bottles Packs of.
500 ml: 6 glass or 6 and 10 polypropylene bottles.
Not all presentations are marketed.
Special precautions for disposal and other handling
Instructions for Use/Handling
Like all parenteral products, OMNIPAQUE should be inspected visually for particulate matter, discoloration and the integrity of the container prior to use. The product should be drawn into the syringe immediately before use. Vials are intended for single use only, any unused portions must be discarded.
The 500 ml contrast medium bottles should only be used in connection with auto injectors/pumps approved for this volume. A single piercing procedure should be used.
The line running from the auto injector/pump to the patient must be exchanged after each patient. Any unused portions of the contrast medium remaining in the bottle and all connecting tubes must be discarded at the end of the day. When convenient, smaller bottles can also be used. Instructions from the manufacturer of the auto injector/pump must be followed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.