OPDUALAG Concentrate for solution for infusion Ref.[50204] Active ingredients: Nivolumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, monoclonal antibodies
ATC code: L01FY02
Mechanism of action
Opdualag is a fixed-dose combination (FDC) of nivolumab, a programmed death-1 inhibitor (anti-PD-1) and relatlimab, a lymphocyte-activation gene-3 inhibitor (anti-LAG-3).
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumours, and signalling through this pathway can contribute to inhibition of active T cell immune surveillance of tumours. Nivolumab is a human IgG4 monoclonal antibody that binds to the PD-1 receptor, blocks interaction with its ligands PD-L1 and PD-L2 and reduces PD-1 pathway-mediated inhibition of the immune response, including the anti-tumour immune response. In syngeneic mouse tumour models, blocking PD-1 activity resulted in decreased tumour growth.
Relatlimab is a human IgG4 monoclonal antibody that binds to the LAG-3 receptor, blocks its interaction with ligands, including MHC II, and reduces LAG-3 pathway-mediated inhibition of the immune response. Antagonism of this pathway promotes T cell proliferation and cytokine secretion.
The combination of nivolumab (anti-PD-1) and relatlimab (anti-LAG-3) results in increased T-cell activation compared to the activity of either antibody alone. In murine syngeneic tumour models, LAG-3 blockade potentiates the anti-tumour activity of PD-1 blockage, inhibiting tumour growth and promoting tumour regression.
Clinical efficacy and safety
Randomised phase 2/3 study of nivolumab in combination with relatlimab vs. nivolumab in patients with previously untreated metastatic or unresectable melanoma (CA224047)
The safety and efficacy of nivolumab in combination with relatlimab for the treatment of patients with previously untreated metastatic or unresectable melanoma were evaluated in a phase 2/3, randomised, double-blinded study (CA224047). The study included patients with ECOG performance status score 0 or 1, and histologically confirmed stage III (unresectable) or stage IV melanoma per American Joint Committee on Cancer (AJCC) version 8. Patients were allowed to have received prior adjuvant or neoadjuvant melanoma therapy (anti-PD-1, anti-CTLA-4, or BRAF-MEK therapy was allowed as long as there was at least 6 months between the last dose of therapy and date of recurrence; interferon therapy was allowed as long as the last dose was at least 6 weeks prior to randomisation). Patients with active autoimmune disease, a history of myocarditis, elevated troponin levels ≥2 times ULN, or ECOG performance status score ≥2, medical conditions requiring systemic treatment with moderate or high dose corticosteroids or immunosuppressive medicinal products, uveal melanoma, and active or untreated brain or leptomeningeal metastases were excluded from the study (see section 4.4).
A total of 714 patients were randomised to receive either nivolumab in combination with relatlimab (n=355), or nivolumab (n=359). Patients in the combination arm received 480 mg nivolumab/160 mg relatlimab over 60 minutes every 4 weeks. Patients in the nivolumab arm received nivolumab 480 mg every 4 weeks. Randomisation was stratified by tumour PD-L1 (≥1% vs. <1) using PD-L1 IHC 28-8 pharmDx test, and LAG-3 expression (≥1% vs. <1) as determined by an analytically validated LAG-3 IHC assay, BRAF V600 mutation status, and M stage per the AJCC version 8 staging system (M0/M1any[0] vs. M1any[ 1 ]). Patients were treated until disease progression or unacceptable toxicity. Tumour assessments, according to the Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1, were conducted 12 weeks after randomisation and continued every 8 weeks up to 52 weeks and then every 12 weeks until disease progression or treatment discontinuation, whichever occurred later. The primary efficacy outcome measure was progression-free survival determined by Blinded Independent Central Review (BICR). The secondary efficacy outcome measures were overall survival (OS), and overall response rate (ORR) by BICR. The hierarchical statistical testing order was PFS followed by OS and then ORR. The primary and secondary outcome measures were evaluated in the intention to treat (ITT) population. No formal testing of ORR was conducted since the formal comparison of OS was not statistically significant.
Baseline characteristics in the ITT population were balanced between the two groups. The median age was 63 years (range: 20-94) with 47% ≥ 65 years of age and 19% ≥75 years of age. The majority of patients were white (97%) and male (58%). Baseline ECOG performance status was 0 (67%) or 1 (33%). The majority of the patients had AJCC Stage IV disease (92%); 38.9% had M1c, 2.4% had M1d disease, 8.7% had prior systemic therapies, 36% had a baseline LDH level greater than ULN at study entry. Thirty nine percent of patients had BRAF mutation-positive melanoma, 75% had LAG-3 ≥1% and 41% of patients had PD-L1 ≥1% tumour cell membrane expression. Among patients with quantifiable tumour PD-L1 expression, the distribution of patients was balanced across the two treatment groups. The demographics and baseline disease characteristics in patients with PD-L1 expression <1% were generally balanced between the treatment arms.
At primary analysis in the ITT population, with median follow-up of 13.21 months (range: 0-33.1 months), a statistically significant improvement in PFS was observed with a median PFS of 10.12 months in the nivolumab in combination with relatlimab group as compared with 4.63 months in the nivolumab group (HR = 0.75, 95% CI: 0.62, 0.92; p = 0.0055). At the time of the pre-specified final OS analysis in the ITT population, with median follow up of 19.3 months, OS was not statistically significant (HR = 0.80, 95% CI: 0.64, 1.01).
Pre-specified subgroup analysis by PD-L1 expression <1%
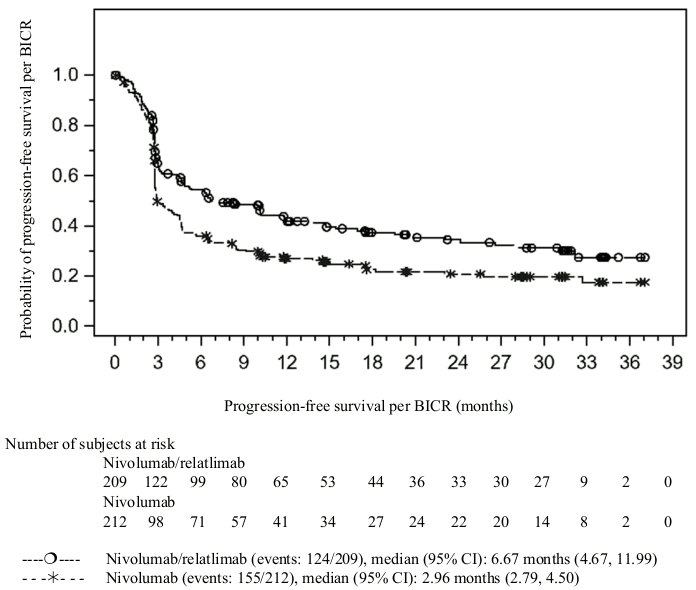
The key efficacy results for the subgroup of patients with tumour PD-L1 expression <1% from an exploratory analysis with median follow-up of 17.78 months (range: 0.26-40.64 months) are summarised in Table 3.
Table 3. Efficacy results in patients with PD-L1 <1% tumour cell expression (CA224047):
| nivolumab + relatlimab (n=209) | nivolumab (n=212) | |
|---|---|---|
| Progression-free survival | ||
| Hazard ratio (95% CI)a | 0.68 (0.53, 0.86) | |
| Median in months (95% CI) | 6.7 (4.7, 12.0) | 3.0 (2.8, 4.5) |
| Rate (95% CI) at 12 months | 42.3 (35.1, 49.4) | 26.9 (20.9, 33.3) |
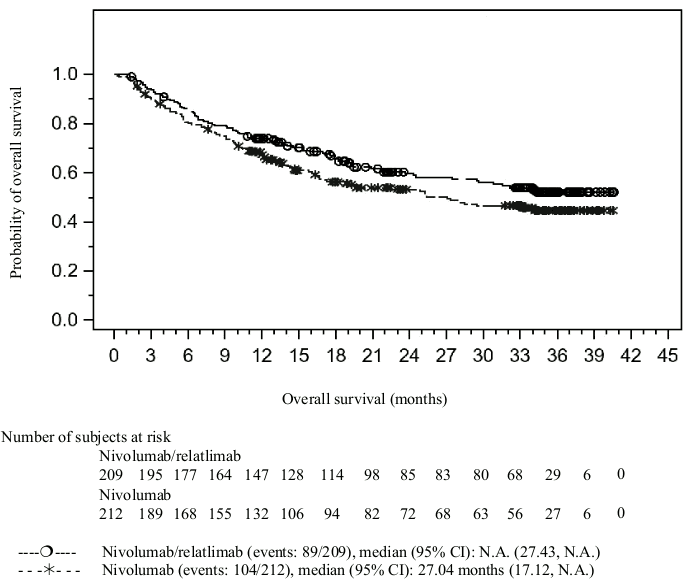
| Overall survivalb | ||
| Hazard ratio (95% CI)a | 0.78 (0.59, 1.04) | |
| Median in months (95% CI) | NR (27.4, NR) | 27.0 (17.1, NR) |
| Rate (95% CI) at 12 months | 73.9 (67.4, 79.4) | 67.4 (60.6, 73.3) |
| Rate (95% CI) at 24 months | 59.6 (52.2, 66.2) | 53.1 (45.8, 59.9) |
| Overall response rate (%) | 36.4 | 24.1 |
| (95% CI) | (29.8, 43.3) | (18.5, 30.4) |
| Complete response rate (%) | 25 (12.0) | 20 (9.4) |
| Partial response rate (%) | 51 (24.4) | 31 (14.6) |
| Stable disease rate (%) | 41 (19.6) | 31 (14.6) |
a Hazard ratio based on unstratified Cox proportional hazards model.
b OS results are not yet mature.
Median extent of follow-up: 17.78 months.
NR = not reached.
The Kaplan-Meier curves for PFS and OS in patients with tumour cell PD-L1 expression <1% are presented in Figures 1 and 2, respectively.
Figure 1. Kaplan-Meier curves of PFS in patients with PD-L1 <1% tumour cell expression (CA224047):
Figure 2. Kaplan-Meier curves of OS in patients with PD-L1 <1% tumour cell expression (CA224047):
5.2. Pharmacokinetic properties
The pharmacokinetics (PK) of relatlimab following the administration of nivolumab in combination with relatlimab was characterised in patients with various cancers who received relatlimab doses of 20 to 800 mg every 2 weeks and 160 to 1440 mg every 4 weeks either as a monotherapy or in combination with nivolumab doses of 80 or 240 mg every 2 weeks or 480 mg every 4 weeks.
Steady-state concentrations of relatlimab were reached by 16 weeks with an every 4-week regimen and the systemic accumulation was 1.9-fold. The average concentration (Cavg) of relatlimab after the first dose increased dose proportionally at doses ≥160 mg every 4 weeks.
Table 4. Geometric mean (CV%) of nivolumab and relatlimab steady-state exposures following 480 mg nivolumab and 160 mg relatlimab fixed-dose combination every 4 weeks:
| Cmax (μg/mL) | Cmin (μg/mL) | Cavg (μg/mL) | |
|---|---|---|---|
| Relatlimab | 62.2 (30.1) | 15.3 (64.3) | 28.8 (44.8) |
| Nivolumab | 187 (32.9) | 59.7 (58.6) | 94.4 (43.3) |
Based on population PK analyses, the nivolumab and relatlimab FDC infusion duration of 30 min and 60 min were predicted to produce similar (<1% different) exposures of nivolumab and relatlimab.
In CA224047, the nivolumab geometric mean Cmin at steady state in the nivolumab in combination with relatlimab arm was similar to the nivolumab arm with a geometric mean ratio of 0.931 (95% CI: 0.855-1.013).
Distribution
The geometric mean value (CV%) for nivolumab volume of distribution at steady state is 6.65 L (19.2%) and relatlimab is 6.65 L (19.8%).
Biotransformation
Nivolumab and relatlimab are therapeutic mAb IgG4 that are expected to be catabolised into small peptides, amino acids, and small carbohydrates by lysosome or receptor-mediated endocytosis.
Elimination
Nivolumab clearance is 21.1% lower [geometric mean (CV%), 7.57 mL/h (40.1%)] at steady state than that after the first dose [9.59 mL/h (40.3%)] and the terminal half-life (t1/2) is 26.5 days (36.4%).
Relatlimab clearance is 9.7% lower [geometric mean (CV%), 5.48 mL/h (41.3%)] at steady state than that after the first dose [6.06 mL/h (38.9%)]. Following administration of relatlimab 160 mg and nivolumab 480 mg administered every 4 weeks, the geometric mean (CV%) effective half-life (t1/2) of relatlimab is 26.2 days (37%).
Special populations
A population PK analysis suggested that the following factors had no clinically important effect on the clearance of nivolumab and relatlimab: age (range: 17 to 92 years), sex, [male (1056) and female (657)], or race [Caucasian (1655), African American (167) and Asian (41)]. The body weight (range: 37 to 170 kg) was a significant covariate on the nivolumab and relatlimab PK, however, there is no clinically relevant impact based on exposure-response analysis.
Paediatric population
Limited data suggest that nivolumab clearance and volume of distribution in adolescent subjects with solid tumours were 36% and 16% lower, respectively, than those of adult reference patients. It is unknown if the same holds for melanoma patients and if relatlimab clearance and volume of distribution are also lower in adolescents than adults. However, based on population PK simulations, the exposure of nivolumab and relatlimab in adolescents weighing at least 30 kg are expected to result in similar safety and efficacy to that of adults of the same weight, at the same recommended dose.
Renal impairment
The effect of renal impairment on the clearance of nivolumab and relatlimab was evaluated by a population PK analysis in patients with mild or moderate renal impairment compared to patients with normal renal function. No clinically important differences in the clearance of nivolumab or relatlimab were found between patients with renal impairment and patients with normal renal function.
Hepatic impairment
The effect of hepatic impairment on the clearance of nivolumab and relatlimab was evaluated by population PK analysis in patients with mild hepatic impairment (total bilirubin [TB] less than or equal to upper limit of normal [ULN] and AST greater than ULN or TB greater than 1 to 1.5 times ULN and any AST) or moderate hepatic impairment (TB greater than 1.5 to 3 times ULN and any AST) compared to patients with normal hepatic function. No clinically important differences in the clearance of nivolumab or relatlimab were found between patients with hepatic impairment and patients with normal hepatic function.
Immunogenicity
The observed low incidence rate of treatment emergent anti-nivolumab antibody and treatment emergent anti-relatlimab antibody had no effects on PK of nivolumab and relatlimab.
5.3. Preclinical safety data
Nivolumab in combination with relatlimab
No animal studies were conducted with nivolumab in combination with relatlimab to evaluate potential carcinogenicity, genotoxicity or reproductive and developmental toxicity.
In a 1-month study in monkeys dosed with nivolumab and relatlimab, inflammation within the central nervous system (choroid plexus, vasculature, meninges, spinal cord) and the reproductive tract (epididymis, seminal vesicles and testes) was observed. Although safety margins were not established for these effects with the combination, they ocurred at doses that suppose exposure levels significantly higher (13 folds for nivolumab and 97 folds for relatlimab) than those reached in patients.
Relatlimab
There are no available animal data on effect of relatlimab on pregnancy and reproduction. In a embryo-foetal toxicity study in mice using murine anti-LAG-3 antibodies, no maternal or developmental effects were observed. The effects of relatlimab on prenatal and postnatal development have not been evaluated; however, based on the mechanism of action, blockade of LAG-3 with relatlimab can have a similar negative effect as nivolumab on pregnancy. There were no fertility studies performed with relatlimab.
Nivolumab
Blockade of the PD-1/PD-L1 pathway has been shown in murine models of pregnancy to disrupt tolerance to the foetus and to increase foetal loss. The effects of nivolumab on prenatal and postnatal development were evaluated in monkeys that received nivolumab twice weekly from the onset of organogenesis in the first trimester through delivery, at exposure levels either 8 or 35 times higher than those observed at the clinical dose of 3 mg/kg of nivolumab (based on AUC). There was a dose-dependent increase in foetal losses and increased neonatal mortality beginning in the third trimester.
The remaining offspring of nivolumab-treated females survived to scheduled termination, with no treatment-related clinical signs, alterations to normal development, organ-weight effects, or gross and microscopic pathology changes. Results for growth indices, as well as teratogenic, neurobehavioral, immunological, and clinical pathology parameters throughout the 6-month postnatal period were comparable to the control group. However, based on their mechanism of action, foetal exposure to nivolumab, and, similarly, relatlimab, may increase the risk of developing immune-related disorders or altering the normal immune response and immune-related disorders have been reported in PD-1 and PD-1/LAG-3 knockout mice. Fertility studies have not been performed with nivolumab.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.