OPFOLDA Hard capsule Ref.[51043] Active ingredients: Miglustat
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Amicus Therapeutics Europe Limited, Block 1, Blanchardstown Corporate Park, Ballycoolin Road, Blanchardstown, Dublin, D15 AKK1, Ireland e-mail: info@amicusrx.co.uk
4.1. Therapeutic indications
Opfolda (miglustat) is an enzyme stabiliser of cipaglucosidase alfa long-term enzyme replacement therapy in adults with late-onset Pompe disease (acid α-glucosidase [GAA] deficiency).
4.2. Posology and method of administration
Treatment should be supervised by a physician experienced in the management of patients with Pompe disease or other inherited metabolic or neuromuscular diseases.
Miglustat 65 mg hard capsules must be used in combination with cipaglucosidase alfa. The summary of product characteristics (SmPC) for cipaglucosidase alfa should be consulted before taking miglustat.
Posology
The recommended dose is to be taken orally every other week in adults aged 18 years and older and is based on body weight:
- For patients weighing ≥50 kg, the recommended dose is 260 mg (4 capsules of 65 mg).
- For patients weighing ≥40 kg to <50 kg, the recommended dose is 195 mg (3 capsules of 65 mg).
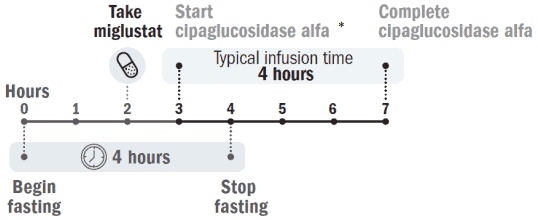
Miglustat 65 mg hard capsules should be taken approximately 1 hour but no more than 3 hours before the start of the cipaglucosidase alfa infusion.
Figure 1. Dose timeline:
* Miglustat 65 mg hard capsules should be taken approximately 1 hour but no more than 3 hours before the start of the cipaglucosidase alfa infusion.
Patient response to treatment should be routinely evaluated based on a comprehensive evaluation of all clinical manifestations of the disease. In case of an insufficient response or intolerable safety risks, discontinuation of miglustat 65 mg hard capsules in combination with cipaglucosidase alfa treatment should be considered. Both medicinal products should either be continued or discontinued.
Missed dose
If the miglustat dose is missed, treatment should occur as soon as possible. If it is not taken, do not start the cipaglucosidase alfa infusion. Cipaglucosidase alfa infusion can start 1 hour after miglustat is taken.
Special populations
Renal and hepatic impairment
The safety and efficacy of miglustat in combination with cipaglucosidase alfa therapy have not been evaluated in patients with renal and/or hepatic impairment. When administering every other week, increased plasma miglustat exposure as a result of moderate or severe renal or hepatic impairment is not expected to appreciably impact cipaglucosidase alfa exposures and is not anticipated to affect efficacy and safety of cipaglucosidase alfa in a clinically meaningful manner. No dose adjustment is required in patients with renal or hepatic impairment.
Elderly
There is limited experience with the use of miglustat in combination with cipaglucosidase alfa therapy in patients above the age of 65 years old. There is no dose adjustment required in elderly patients.
Paediatric population
The safety and efficacy of miglustat in combination with cipaglucosidase alfa therapy in paediatric patients less than 18 years old have not yet been established. No data are available.
Method of administration
Miglustat is for oral use.
Miglustat hard capsule has a crimp to prevent opening the capsule shells, and should be swallowed whole and taken on an empty stomach.
Patients should fast 2 hours before and 2 hours after taking miglustat 65 mg hard capsules (see section 5.2). During this 4-hour fasting period, water, fat-free (skimmed) cow’s milk, and tea or coffee with no cream, sugars, or sweeteners can be consumed. The patient can resume normal eating and drinking 2 hours after taking miglustat.
4.9. Overdose
Symptoms
Leukopenia, granulocytopenia, neutropenia, dizziness, and paraesthesia have been observed in human immunodeficiency virus (HIV) patients receiving miglustat at a dosage of 800 mg/day or higher.
Management
In the event of an overdose, supportive medical care should be provided immediately. Full blood counts should be monitored for reduced white cells.
6.3. Shelf life
3 years.
6.4. Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5. Nature and contents of container
40 mL high density polyethylene (HDPE) bottle with 33 mm white child resistant polypropylene cap with label. Bottle opening is sealed with an induction sealed foil liner.
Bottles of 4 and 24 capsules.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.