ORPHENADRINE CITRATE Solution for injection Ref.[50744] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
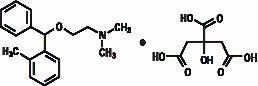
Orphenadrine citrate is the citrate salt of orphenadrine (±)-N,N-dimethyl-2-[(omethylα-phenylbenzyl)oxy]-ethylamine citrate (1:1). It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol.
Each vial contains 60 mg of orphenadrine citrate in aqueous solution. Each vial also contains: sodium metabisulfite, 2 mg; sodium chloride, 5.8 mg; sodium hydroxide, to adjust pH; and water for injection, q.s. to 2 mL.
The structural formula is:
C18H23NO•C6H8O7 MW 461.50
| How Supplied |
|---|
|
Orphenadrine Citrate Injection, USP is supplied as follows: Cartons of 10 (NDC 0641-6182-10) 2 mL vials, each vial containing 60 mg of orphenadrine citrate in aqueous solution. Manufactured by: Hikma Pharmaceuticals USA Inc., Berkeley Heights, NJ 07922 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.