ORUDIS SR Capsule Ref.[50303] Active ingredients: Ketoprofen
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2022 Publisher: sanofi-aventis australia pty ltd, 12-24 Talavera Road, Macquarie Park, NSW 2113, Toll Free Number (medical information): 1800 818 806, Email: medinfo.australia@sanofi.com
Product name and form
ORUDIS SR Ketoprofen.
| Pharmaceutical Form |
|---|
|
Orudis SR Capsules: modified release, hard gelatin capsule. Clear pink base & white opaque cap. Base and cap printed ‘ORUDIS SR 200’ and contains off-white to cream spherical pellets. |
Qualitative and quantitative composition
Each Orudis SR capsule contains ketoprofen 200 mg.
For the full list of excipients, see Section 6.1 List of excipients.
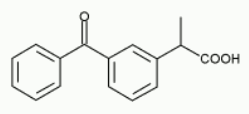
Ketoprofen is DL-2-(3-benzoylphenyl) propionic acid. It is a white or off-white powder with melting point of about 93°C. MW: 254.3. Ketoprofen is very slightly soluble in water at 20°C, 2% soluble in dimethylformide and readily soluble in benzene, ethanol, chloroform, acetone and ethyl acetate at 20°C.
Chemical structure
CAS number: 22071-15-4
| Active Ingredient |
|---|
|
Ketoprofen is a non-steroidal anti-inflammatory drug. It has anti-inflammatory and analgesic actions. |
| List of Excipients |
|---|
|
Orudis SR Capsules: Erythrosine, ethylcellulose, gelatin, non-pareil beads (PI 1014) (sucrose and maize starch), OPACODE monogramming ink S-1-20952 BLUE (PI 12300), shellac, colloidal anhydrous silica, sodium lauryl sulfate, purified talc and titanium dioxide. |
Pack sizes and marketing
Orudis SR 200 mg Capsules are available in the following presentations: Blister pack of 4*, 28, 30* and 100* capsules.
* non-marketed pack sizes.
Marketing authorization holder
sanofi-aventis australia pty ltd, 12-24 Talavera Road, Macquarie Park, NSW 2113, Toll Free Number (medical information): 1800 818 806, Email: medinfo.australia@sanofi.com
Marketing authorization dates and numbers
21 October 1991
Drugs
| Drug | Countries | |
|---|---|---|
| ORUDIS | Australia, Spain, Finland, Italy, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.