OVIDREL Solution for injection Ref.[10894] Active ingredients: Choriogonadotropin alpha
Source: FDA, National Drug Code (US) Revision Year: 2020
3. Indications and Usage
Ovidrel PreFilled Syringe (choriogonadotropin alfa injection) is indicated for the induction of final follicular maturation and early luteinization in infertile women who have undergone pituitary desensitization and who have been appropriately pretreated with follicle stimulating hormones as part of an Assisted Reproductive Technology (ART) program such as in vitro fertilization and embryo transfer. Ovidrel PreFilled Syringe is also indicated for the induction of ovulation (OI) and pregnancy in anovulatory infertile patients in whom the cause of infertility is functional and not due to primary ovarian failure.
Selection of Patients:
- Before treatment with gonadotropins is instituted, a thorough gynecologic and endocrinologic evaluation must be performed. This should include an assessment of pelvic anatomy. Patients with tubal obstruction should receive Ovidrel PreFilled Syringe only if enrolled in an in vitro fertilization program.
- Primary ovarian failure should be excluded by the determination of gonadotropin levels.
- Appropriate evaluation should be performed to exclude pregnancy.
- Patients in later reproductive life have a greater predisposition to endometrial carcinoma as well as a higher incidence of anovulatory disorders. A thorough diagnostic evaluation should always be performed in patients who demonstrate abnormal uterine bleeding or other signs of endometrial abnormalities before starting FSH and Ovidrel PreFilled Syringe therapy.
- Evaluation of the partner’s fertility potential should be included in the initial evaluation.
10. Dosage and Administration
For Subcutaneous Use Only.
Infertile Women Undergoing Assisted Reproductive Technologies (ART)
Ovidrel PreFilled Syringe 250 µg should be administered one day following the last dose of the follicle stimulating agent. Ovidrel PreFilled Syringe should not be administered until adequate follicular development is indicated by serum estradiol and vaginal ultrasonography. Administration should be withheld in situations where there is an excessive ovarian response, as evidenced by clinically significant ovarian enlargement or excessive estradiol production.
Infertile Women Undergoing Ovulation Induction (OI)
Ovidrel PreFilled Syringe should not be administered until adequate follicular development is indicated by serum estradiol and vaginal ultrasonography.
Ovidrel PreFilled Syringe 250 µg should be administered one day following the last dose of the follicle stimulating agent.
Ovidrel PreFilled Syringe administration should be withheld in situations where there is an excessive ovarian response, as evidenced by multiple follicular development, clinically significant ovarian enlargement or excessive estradiol production.
Directions for Administration of Ovidrel Prefilled Syringe
Ovidrel PreFilled Syringe is intended for a single subcutaneous injection. Any unused material should be discarded.
Ovidrel PreFilled Syringe may be self-administered by the patient. Follow the directions below for injecting Ovidrel PreFilled Syringe.
Step 1: Wash your hands thoroughly with soap and water.
Step 2: Carefully clean the injection site.
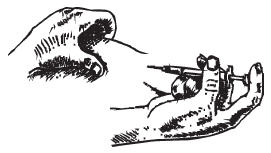
Make yourself comfortable by sitting or lying down. Carefully clean the injection site on the stomach with an alcohol wipe and allow it to air-dry.
Step 3: Administer your injection.
Carefully remove the needle cap from the syringe. Do not touch the needle or allow the needle to touch any surface. Inject the prescribed dose as directed by your doctor, nurse or pharmacist.
Step 4: Gently withdraw the needle.
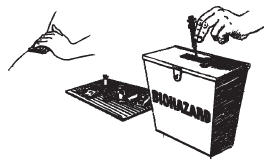
Discard the needle and syringe into your safety container. Place gauze over the injection site. If any bleeding occurs, apply gentle pressure. If bleeding does not stop within a few minutes, place a clean piece of gauze over the injection site and cover it with an adhesive bandage.
Step 5: Storage and clean up.
Remember that your injection materials must be kept sterile and cannot be reused.
12. Storage and Handling
The Ovidrel PreFilled Syringe must be stored refrigerated between 2-8°C (36-46°F) before being dispensed to the patient. Patients should store the pre-filled syringe refrigerated to allow the product to be used until the expiry date shown on the syringe or carton. The Ovidrel PreFilled Syringe may be stored by the patient for no more than 30 days at room temperature (up to 25°C (77°F) but must be used within those 30 days.
Protect from light.
Store in original package. Discard unused material.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.