OXERVATE Eye drops, solution Ref.[7635] Active ingredients: Cenegermin
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Dompé farmaceutici S.p.A., Via Santa Lucia, 6, 20122 Milano Italy, Tel. +39 02 583831, Fax +39 02 58383215, E-mail: info@dompe.com
4.1. Therapeutic indications
Treatment of moderate (persistent epithelial defect) or severe (corneal ulcer) neurotrophic keratitis in adults.
4.2. Posology and method of administration
Treatment should be initiated and supervised by an ophthalmologist or a healthcare professional qualified in ophthalmology.
Posology
Adults
The recommended dose is one drop of OXERVATE in the conjunctival sac of the affected eye(s), 6 times a day at 2 hourly intervals, starting from the morning and within 12 hours. Treatment should be continued for eight weeks.
Patients with an eye infection should be treated before starting therapy with OXERVATE (see section 4.4).
If a dose is missed, treatment should be continued as normal, at the next scheduled administration. The missed dose can be administered later, within the 12 hours shelf-life of the daily vial. Patients should be advised not to instil more than one drop in the affected eye(s) during any administration.
Special populations
Elderly
No dose adjustment is required in patients 65 years of age and older.
Hepatic and renal impairment
The medicinal product has not been studied in patients with hepatic or renal impairment. However, no dose adjustment is considered necessary in these populations.
Paediatric population
The safety and efficacy of this medicinal product in children and adolescents below the age of 18 years have not been established. No data are available.
Method of administration
For ocular use only.
Precautions to be taken before administering the medicinal product
Patients should be instructed to wash their hands before use.
OXERVATE should only be administered using the associated delivery system (vial adapter and pipettes), according to the instructions presented in section 6.6. An individual pipette should be used per application.
If more than one topical ophthalmic product is being used, the eye drops must be administered at least 15 minutes apart, to avoid diluting the other product. If eye ointment, gel or other viscous eye drops are used, they should be administered 15 minutes following OXERVATE treatment (see also section 4.5).
In case of concomitant use with contact lenses, see section 4.4.
For instructions on preparation and handling of the medicinal product before administration, see section 6.6.
4.9. Overdose
A topical overdose is not likely to occur or to be associated with toxicity. A topical overdose of cenegermin may be flushed from the eye(s) with lukewarm water.
6.3. Shelf life
Unopened vial:
2 years.
Opened vial:
Once opened, the product must be stored below 25°C and used within 12 hours at 25°C.
From a microbiological point of view, the method of opening (i.e. by connecting the vial adapter to the vial) precludes the risk of microbial contamination.
6.4. Special precautions for storage
OXERVATE vials
Pharmacy (unopened vial):
The weekly carton containing the vials must be stored in a freezer (-20°C ± 5°C).
Patient (unopened vial):
The patient will receive a weekly carton including 7 vials of OXERVATE in an insulated pack. As soon as the patient is at home (and no later than 5 hours from when the patient receives the product at the pharmacy), the weekly carton should be placed into the refrigerator, at 2-8°C. It should be noted that the frozen medicinal product received from the pharmacy could need up to 30 minutes for thawing.
Patient (opened vial):
An individual multi-dose vial of OXERVATE is to be removed from the fridge for use over the course of a single day. Each opened vial can be stored in the fridge or below 25°C, but must be used within 12 hours.
After this period of time the vial contents should be discarded irrespective of whether some residual product remains in the vial.
6.5. Nature and contents of container
1 ml OXERVATE solution in sterile, preservative-free multi-dose Type I glass vials, closed with a rubber stopper and an aluminium overseal with a polypropylene flip-off cap, presented in cardboard cartons.
Pack size: 7 multi-dose vials per carton
The patient will receive a weekly carton containing 7 vials of OXERVATE.
This medicinal product should only be used with specific vial adapters and disposable devices (pipettes) that will be provided separately from the weekly OXERVATE carton. 7 vial adapters (i.e. 1 per day), 42 pipettes (i.e. 6 per day) and 42 disinfectant wipes (i.e. 6 per day) sufficient to administer the medicinal product for one week will be provided separately, together with a dose recording card. Extra adapter (1), pipettes (3) and wipes (3) will also be provided as spares.
6.6. Special precautions for disposal and other handling
The patient will receive a weekly carton containing 7 multi-dose vials of OXERVATE, which should be stored in a refrigerator until the day of use.
The patient will also receive separately vial adapters, pipettes and disinfectant wipes.
An individual multi-dose vial of OXERVATE should be taken from the refrigerator at the same time each morning bearing in mind the 12 hour treatment schedule. The multi-dose vial containing the product should be prepared according to the following instructions:
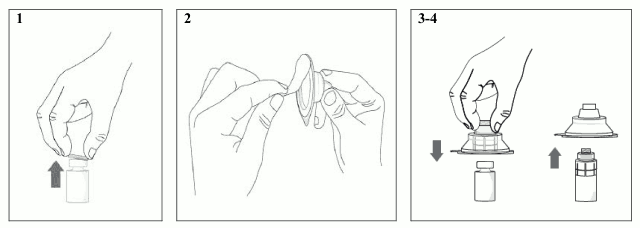
1) With clean freshly-washed hands, place the vial on a steady flat surface and remove the plastic flip-off cap.
2) Peel-off the back of the vial adapter blister pack.
3) Without removing the vial adapter from its blister pack, connect the vial adapter to the vial by firmly pushing the vial adapter down vertically until it snaps into place over the neck of the vial and the spike of the vial adapter pierces through the vial’s rubber stopper. Once the vial adapter has been connected correctly, it should not be removed from the vial.
4) Remove and discard the vial adapter blister pack. Avoid touching the surface of the adapter.
To withdraw and administer each dose of OXERVATE solution, the steps below should be followed:
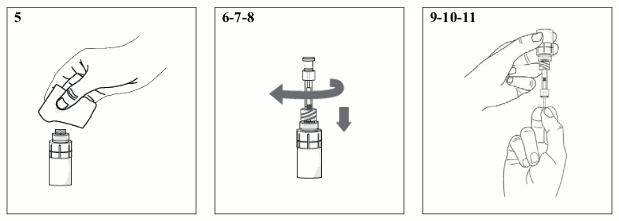
5) Take an individual disinfectant wipe and gently clean the surface of the valve on the luer lock connector of the vial adapter. After cleaning, the valve should be allowed to dry for approximately one minute.
6) Take a pipette and remove it from the protective packaging.
7) Screw the pipette clockwise into the luer lock connector of the vial adapter.
8) Ensure that the pipette plunger is pushed all the way down.
9) Turn the vial upside-down with the pipette connected and gently pull the pipette plunger outwards until it stops, to draw the solution into the pipette (ensure that the plunger has reached the stop point).
10) Check the pipette and confirm that it contains some of the solution. Air bubbles may cause blockage and prevent the pipette from filling properly (especially at first withdrawal). If the pipette is empty, keep the vial with the connected pipette upside-down, push the plunger all the way in and pull it out again.
11) Once it has been correctly filled, unscrew the pipette from the luer lock connector of the vial adapter.
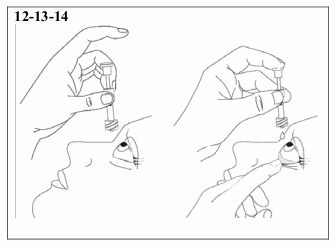
12) Hold the pipette, pointing down, between the middle finger and thumb, tilt the head back and position the pipette above the affected eye. Pull down the lower eyelid. Gently push the pipette plunger in until a single drop is instilled into the conjunctival fornix.
13) Immediately discard the used pipette and wipe after instillation.
14) If a mistake is made and a drop is not instilled into the eye, repeat the steps described above using a new pipette and wipe.
15) Throughout the day, the vial can either be placed back in the fridge after each use or stored below 25°C (with the vial adapter still connected).
The administration instructions above (steps 5 to 15) should be repeated every 2 hours (six times per day) using a new disinfectant wipe and a new pipette each time.
The vial and any remaining solution must be discarded at the end of the day, and no later than 12 hours from the time the vial adapter was connected (irrespective of whether any residual solution remains in the vial).
To ensure accurate dosing every 2 hours, the patient should be advised to set an alarm as a reminder for dosing.
To control that six doses have been taken every day, the patient should be advised to use the weekly dose recording card provided with the delivery system. On that card the patient should track the date of the first use of the weekly supply, the time of the vial opening (i.e. when the vial adapter is connected to the vial), and the time of daily ocular instillations occurring over the week.
A new OXERVATE supply will be issued each week for the duration of the treatment period.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.