OXYCODONE HYDROCHLORIDE Capsule Ref.[10605] Active ingredients: Oxycodone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
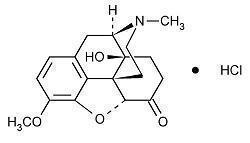
Oxycodone Hydrochloride Capsule is an agonist, available as a hard gelatin capsule 5 mg for oral administration. The chemical name is (5R,9R,13S,14S)-4, 5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride. The molecular weight is 351.82. Its molecular formula is C18H21NO4•HCl, and it has the following chemical structure.
Oxycodone hydrochloride is a white, odorless crystalline powder derived from the opium alkaloid, thebaine. It is soluble in water and slightly soluble in alcohol.
The inactive ingredients in Oxycodone Hydrochloride Capsules, 5 mg include: black iron oxide, colloidal silicon dioxide, FD&C Yellow #6, gelatin, red iron oxide, starch pregelatinized (corn), stearic acid, titanium dioxide, and yellow iron oxide. The imprinting on the hard gelatin capsule is black ink.
| Dosage Forms and Strengths |
|---|
|
Oxycodone Hydrochloride Capsules, USP. Capsules 5 mg: Each capsule has a light brown opaque cap, peach opaque body with black imprint “K 23” on both cap and body, filled with powder containing 5 mg of oxycodone hydrochloride, USP. |
| How Supplied |
|---|
|
Oxycodone Hydrochloride Capsule 5 mg is a hard gelatin capsule with a light brown opaque cap, peach opaque body with black imprint “K 23” on both cap and body, filled with powder, supplied as: NDC 10702-023-06: Bottle of 60 Capsules Dispense in a tight, light-resistant container. Manufactured by: KVK-Tech, Inc., 110 Terry Drive, Newtown, PA 18940 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.