PALYNZIQ Solution for injection Ref.[10154] Active ingredients: Pegvaliase
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Pegvaliase-pqpz is a PEGylated phenylalanine ammonia lyase (PAL) enzyme that converts phenylalanine to ammonia and trans‑cinnamic acid. It substitutes for the deficient phenylalanine hydroxylase (PAH) enzyme activity in patients with PKU and reduces blood phenylalanine concentrations.
12.2. Pharmacodynamics
Palynziq treatment of adult patients with PKU resulted in the reduction of blood phenylalanine concentrations from pre-treatment baseline [see Clinical Studies (14)]. The reduction of blood phenylalanine concentrations diminished with decreased pegvaliase‑pqpz plasma concentrations.
12.3. Pharmacokinetics
The pharmacokinetics of pegvaliase‑pqpz exhibit high inter-patient and intra-patient variability due to the heterogeneity of the immune response in adult patients with PKU. Higher antibody titers correlated with higher apparent clearance of pegvaliase‑pqpz. In the first eight weeks of induction/titration treatment, plasma pegvaliase‑pqpz concentrations were low to not measurable. At steady state during maintenance treatment with Palynziq 20 mg, 40 mg, and 60 mg subcutaneously once daily, the mean ± SD (range) plasma trough pegvaliase‑pqpz concentrations were: 11.2 ± 9.0 (0.21 to 29.6) mg/L, 10.4 ± 12.7 (0.18 to 43.1) mg/L and 4.8 ± 10.7 (0 to 39.7) mg/L, respectively. The following pharmacokinetic parameters were observed in adult patients with PKU treated with Palynziq at maintenance dosages of 20 mg once daily and 40 mg once daily.
Absorption
The median Tmax was approximately 8 hours. The mean ± SD (range) peak concentration (Cmax) at steady state was: 14.0 ± 16.3 (0.26 to 68.5) mg/L and 16.7 ± 19.5 (0.24 to 63.8) mg/L, respectively.
Distribution
The mean ± SD (range) apparent volume of distribution was 26.4 ± 64.8 (1.8 to 241) L and 22.2 ± 19.7 (3.1 to 49.5) L, respectively.
Elimination
The mean ± SD (range) apparent clearance at steady state was 0.39 ± 0.87 (0.018 to 3.66) L/h and 1.25 ± 2.46 (0.034 to 8.88) L/h, respectively. The mean ± SD (range) half-life was 47 ± 42 (14 to 132) hours and 60 ± 45 (14 to 127) hours, respectively.
Metabolism
The metabolism of phenylalanine ammonia lyase is expected to occur via catabolic pathways and be degraded into small peptides and amino acids.
Excretion
The route of elimination of pegvaliase‑pqpz has not been studied in humans.
13.1. Carcinogensis, Mutagenesis, Impairment of Fertility
Carcinogenicity and genotoxicity studies have not been performed with pegvaliase‑pqpz. Based on its mechanism of action, pegvaliase‑pqpz is not expected to be tumorigenic.
Pegvaliase-pqpz produced impaired fertility in female rats at 20 mg/kg/day subcutaneously (13 times the human steady-state AUC at the maximum recommended daily dose), as indicated by decreases in corpora lutea, implantations, and litter size. These effects were associated with maternal toxicity (decreased body weight, ovarian weight, and food consumption). No effects on mating or fertility were observed in female rats with 8 mg/kg/day subcutaneously (2.8 times the human steady-state AUC at the maximum recommended daily dose) or in male rats with 20 mg/kg/day subcutaneously.
13.2 Animal Toxicology and/or Pharmacology
In rats without PKU treated with pegvaliase-pqpz, dose-dependent vacuolation in multiple organs and tissues was observed in the 4‑ and 26‑week repeat dose toxicity studies at doses of 8 mg/kg subcutaneously or greater administered twice weekly (less than the human steady state AUC at the maximum recommended daily dose). Vacuolation occurred in renal tubule cells and in histiocytic cells of the liver, spleen, testes, adrenal cortex, mesenteric lymph node, and mandibular lymph node. Vacuolation in histiocytes of the affected organs and tissues persisted after cessation of treatment. The vacuolation observed in these studies was not associated with organ‑related toxicities as determined by clinical chemistry/urinalysis and histopathological examination. The clinical significance of these findings and functional consequences are unknown.
In the 39‑week repeat dose toxicity study in monkeys, pegvaliase-pqpz 3 mg/kg subcutaneously twice weekly (2 times the human steady state AUC at the maximum recommended daily dose) produced systemic arteritis involving small arteries and arterioles in a wide range of organs and tissues (kidney, urinary bladder, pancreas, gallbladder, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, lung, heart, sciatic nerve, lacrimal gland, mandibular lymph node, epididymis, seminal vesicle, ovary, uterus, cervix, and vagina) and in subcutaneous injection sites. Arteritis was likely due to the immune-mediated response (e.g., immune complex deposition in blood vessels) associated with chronic administration of a foreign protein to the animals. The incidence and severity of systemic arteritis was dose-dependent. The vascular inflammation observed in this study was not associated with organ related toxicities as determined by clinical pathology parameters (hematology, clinical chemistry, and urinalysis) and histopathological examination.
Studies of longer duration in rats and monkeys treated with pegvaliase‑pqpz have not been conducted.
14. Clinical Studies
Study 301: Induction/Titration/Maintenance Treatment
Study 165‑301 (referred to as Study 301, NCT01819727) was an open-label randomized, multi-center study of adults with PKU to assess safety and tolerability of self-administered Palynziq in an induction/titration/maintenance regimen with a target maintenance dose of 20 mg subcutaneously once daily or 40 mg subcutaneously once daily. At Palynziq treatment initiation, 253 patients demonstrated inadequate blood phenylalanine control (blood phenylalanine concentration greater than 600 micromol/L) on existing management, and 8 patients had blood phenylalanine concentrations less than or equal to 600 micromol/L. Existing management options included prior or current restriction of dietary phenylalanine and protein intake, and/or prior treatment with sapropterin dihydrochloride. Patients previously treated with sapropterin dihydrochloride were required to discontinue use at least 14 days prior to the first dose.
The 261 enrolled patients were aged 16 to 55 years (mean: 29 years) and had a baseline mean (range) blood phenylalanine of 1,233 (285, 2330) micromol/L. One hundred forty nine out of 261 (57%) patients were taking medical food at baseline and 41 out of 261 patients (16%) were on a protein-restricted diet at baseline (defined as receiving greater than 75% of total protein intake from medical food). Patients were randomized (1:1) to one of two target maintenance dosage arms: 20 mg once daily or 40 mg once daily. Patients were titrated to reach their randomized target dosage of 20 mg once daily or 40 mg once daily. The duration of titration varied among patients and was based on patient tolerability. Of the 261 enrolled patients, 195 (75%) patients reached their randomized maintenance dosage (103 in the 20 mg once daily arm, 92 in the 40 mg once daily arm). Among the patients who reached their randomized maintenance dosage, patients in the 20 mg once daily randomized arm reached their maintenance dosage at a median time of 10 weeks (range: 9 to 29 weeks) and patients in the 40 mg once daily arm reached their maintenance dosage at a median time of 11 weeks (range: 10 to 33 weeks).
Of the 261 patients who enrolled in Study 301, 54 (21%) patients discontinued treatment during Study 301, 4 patients completed Study 301 and did not continue to Study 165‑302 (referred to as Study 302, NCT01889862), 152 patients continued to the eligibility period of Study 302, and 51 patients continued directly from Study 301 into the long‑term treatment period of Study 302.
Study 302: Efficacy Assessment
A total of 164 adult patients with PKU who were previously-treated with Palynziq (152 patients from Study 301 and 12 patients from other Palynziq trials) enrolled in Study 302 and continued treatment with Palynziq in Study 302 for up to 13 weeks to assess eligibility for randomized withdrawal period.
Randomized Withdrawal Period
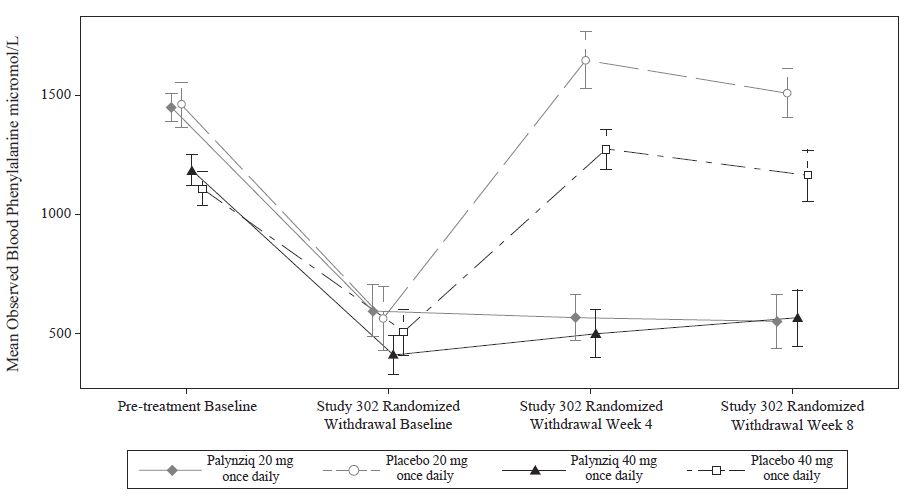
Following this period of up to 13 weeks of additional Palynziq treatment in Study 302, eligibility for entry into the efficacy assessment period (randomized withdrawal period) was determined by whether a patient achieved at least a 20% reduction in blood phenylalanine concentration from pre-treatment baseline (when in previous studies). Eighty‑six out of 164 patients (52%) met this response target and continued into the randomized withdrawal period. In the double-blind, placebo-controlled, randomized withdrawal period, patients were randomized in a 2:1 ratio to either continue their maintenance Palynziq dosage or to receive matching placebo for a total of 8 weeks. The treatment difference in least squares (LS) mean change in blood phenylalanine concentration from the Study 302 randomized withdrawal baseline to randomized withdrawal Week 8 for each randomized study arm is shown in Table 5. Mean blood phenylalanine concentrations at pre-treatment baseline (Study 301 or other Palynziq trials) are also shown in Table 5. At Study 302 randomized withdrawal Week 8, Palynziq‑treated patients (20 mg once daily or 40 mg once daily) maintained their blood phenylalanine concentrations as compared to their randomized withdrawal baseline, whereas patients randomized to matching placebo (20 mg once daily or 40 mg once daily) returned to their pretreatment baseline blood phenylalanine concentrations (Figure 1).
Table 5. Primary Endpoint: LS Mean Change in Blood Phenylalanine Concentration from Randomized Withdrawal Baseline to Week 8 in Adult Patients with PKU – Efficacy Assessment in Study 302:
| Randomized Study Arm | Blood Phenylalanine Concentration (micromol/L) Mean (SD) | LS Mean Change from Study 302 Randomized Withdrawal Baseline to Week 8 (95% CI) | Treatment Difference in LS Mean Change (95% CI) P‑value* | ||
|---|---|---|---|---|---|
| Pre‑treatment Baseline | Study 302 Randomized Withdrawal Baseline | Study 302 Randomized Withdrawal Week 8 | |||
| Palynziq 20 mg once daily | 1450.2 (310.5) n=29 | 596.8 (582.8) n=29 | 553.0 (582.4) n=26† | -23.3 (-156.2, 109.7) | -973.0 (‑1204.2, ‑741.9) p<0.0001 |
| Placebo 20 mg once daily | 1459.1 (354.7) n=14 | 563.9 (504.6) n=14 | 1509.0 (372.6) n=13† | 949.8(760.4, 1139.1) | |
| Palynziq 40 mg once daily | 1185.8 (344.0) n=29 | 410.9 (440.0) n=29 | 566.3 (567.5) n=23† | 76.3 (-60.2, 212.8) | -588.5 (-830.1, -346.9) p<0.0001 |
| Placebo 40 mg once daily | 1108.9 (266.8) n=14 | 508.2 (363.7) n=14 | 1164.4 (343.3) n=10† | 664.8 (465.5, 864.1) | |
* Based on the mixed model repeated measures (MMRM) method, with treatment arm, visit, and treatment arm-by-visit interaction as factors adjusting for baseline blood phenylalanine concentration.
† Patients who did not complete phenylalanine assessment within the window for Week 8 (Day 43 to 56) were excluded.
Figure 1. Observed Mean (SE) Blood Phenylalanine Concentrations Over Time in Adult Patients with PKU in Study 302:
Study 301 and 302 Continuous Treatment
Of 118 patients from Study 301 with a pre‑treatment baseline blood phenylalanine concentration greater than 600 micromol/L who were randomized to and received at least one dose of 20 mg once daily Palynziq, 107 patients, 97 patients, 93 patients, 86 patients, and 77 patients were treated for at least 6 months, 12 months, 18 months, 24 months, and 36 months, respectively. During the continuous treatment period, patients were allowed to dose up to 60 mg once daily based on investigator discretion to achieve blood phenylalanine lowering (for example, to achieve blood phenylalanine concentrations between 120 and 360 micromol/L).
Of the 118 patients, a majority (91 patients, 77%) reached their first response, defined as a blood phenylalanine concentration less than or equal to 600 micromol/L, at a time point prior to 36 months. Of the 91 patients, 9 patients (10%) achieved their first response at a dose less than 20 mg once daily, 44 patients (48%) achieved their first response at a dose of 20 mg once daily, 26 patients (29%) achieved their first response at a dose of 40 mg once daily, and 12 patients (13%) achieved their first response at a dose of 60 mg once daily. Of the 44 patients that achieved their first response at a dose of 20 mg once daily, 36 (82%) achieved it by 24 weeks of treatment. Of the 26 patients that achieved their first response at a dose of 40 mg once daily, 18 (69%) achieved it by 16 weeks of treatment. Of the 12 patients that achieved their first response at a dose of 60 mg once daily, 8 (67%) achieved it by 16 weeks of treatment.
Of the 107 patients treated for at least 6 months, 28 (26%), 18 (17%), and 11 (10%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 6 months of treatment. Of the 97 patients treated for at least 12 months, 47 (48%), 41 (42%), and 31 (32%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 12 months of treatment. Of the 93 patients treated for at least 18 months, 64 (69%), 46 (49%), and 36 (39%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 18 months of treatment. Of the 86 patients treated for at least 24 months, 65 (76%), 57 (66%), and 43 (50%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 24 months of treatment. Of the 77 patients treated for at least 36 months, 58 (75%), 51 (66%), and 37 (48%) had a blood phenylalanine concentration less than or equal to 600, 360, and 120 micromol/L, respectively, at 36 months of treatment.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.