PAMELOR Capsule Ref.[49683] Active ingredients: Nortriptyline
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
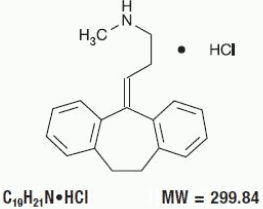
Pamelor (nortriptyline HCl) is 1-propanamine, 3-(10,11-dihydro-5Hdibenzo[a,d] cyclohepten-5-ylidene)N - methyl -, hydrochloride.
The structural formula is as follows:
10 mg, 25 mg, 50 mg, and 75 mg Capsules
Active Ingredient: nortriptyline hydrochloride USP.
10 mg, 25 mg, and 75 mg Capsules
Inactive Ingredients: D&C Yellow #10, FD&C Yellow #6, gelatin, silicone fluid, starch, and titanium dioxide.
50 mg Capsules
Inactive Ingredients: gelatin, silicone fluid, starch, and titanium dioxide.
| How Supplied |
|---|
Pamelor (nortriptyline HCl) Capsules USPPamelor (nortriptyline HCl) Capsules USP, equivalent to 10 mg, 25 mg, 50 mg, and 75 mg base, are available as follows: 10 mg: Light orange opaque cap printed "⌒ PAMELOR 10 mg" in black and white opaque body printed "M" in black. Bottles of 30 - NDC 0406-9910-03 25 mg: Light orange opaque cap printed "⌒ PAMELOR 25 mg" in black and white opaque body printed "M" in black. Bottles of 30 - NDC 0406-9911-03 50 mg: White opaque cap printed "⌒ PAMELOR 50 mg" in black and white opaque body printed "M" in black. Bottles of 30 - NDC 0406-9912-03 75 mg: Light orange opaque cap printed "⌒ PAMELOR 75 mg" in black and light orange opaque body printed "M" in black. Bottles of 30 - NDC 0406-9913-03 Mallinckrodt, the "M" brand mark, the Mallinckrodt Pharmaceuticals logo, and other brands are trademarks of a Mallinckrodt company. Manufactured by: Patheon Inc. Whitby, Ontario, Canada, L1N 5Z5 Manufactured for: SpecGx LLC Webster Groves, MO 63119, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| PAMELOR | Brazil, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.