PENTHROP Inhalation solution Ref.[50526] Active ingredients:
Source: Health Products Regulatory Authority (ZA) Revision Year: 2021 Publisher: Equity Pharmaceuticals (Pty) Ltd., 100 Sovereign Drive, Route 21 Corporate Park, Nellmapius Drive, Irene, Pretoria
4.1. Therapeutic indications
- For emergency relief of pain by self-administration in conscious haemodynamically stable patients with trauma and associated pain, under supervision of healthcare professionals trained in PENTHROP use (see section 4.2).
- For the relief of pain in monitored conscious patients who require analgesia for surgical procedures such as the change of dressings (see section 4.2).
Note: The total maximum dose must not be exceeded (see section 4.2).
4.2. Posology and method of administration
Posology
For use only as an analgesic medicine, see section 4.3.
Dosage (adults)
Up to 6 ml (2 × 3 ml bottles) of PENTHROP per day, vaporised in a PENTHROP Inhaler. If refilling the inhaler with a second bottle of PENTHROP, this should occur only once and must be conducted in a well-ventilated area to reduce environmental exposure to PENTHROP vapour. To maximise safety, the lowest effective dosage of PENTHROP to provide analgesia should be used, particularly for children and the elderly.
The total weekly dose should not exceed 15 ml.
Administration on consecutive days is not recommended.
The cumulative dose received by patients receiving intermittent doses of PENTHROP for painful procedures (such as wound dressings) must be carefully monitored to ensure that the recommended dose of PENTHROP is not exceeded. PENTHROP may cause renal failure if the recommended dose is exceeded.
PENTHROP-associated renal failure is generally irreversible.
Paediatric population
1 to 11 years of age: Up to 3 ml (1 × 3 ml) of PENTHROP per day or 15 ml (5 × 3 ml bottles) per week.
12 to 17 years of age: Up to 6 ml (2 × 3 ml) of PENTHROP per day or 15 ml (5 × 3 ml bottles) per week.
The total weekly dose should not exceed 15 ml.
Administration on consecutive days is not recommended.
Method of administration
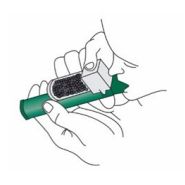
PENTHROP is self-administered, under observation and supervision (and assisted if necessary) by a person trained in its administration, using the hand held PENTHROP Inhaler. Instructions on the preparation of the PENTHROP Inhaler and correct administration are provided in Figure 1.
Only PENTHROP is to be used in the PENTHROP Inhaler. No other inhalational anaesthetics or inhalational analgesic may be used.
Figure 1. How to use the PENTHROP Inhaler
1. Ensure the Activated Carbon (AC) Chamber (where applicable) is inserted into the dilutor hole on the top of the PENTHROP Inhaler.
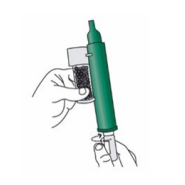
2. Remove the cap of the bottle by hand. Alternatively, use the base of the PENTHROP Inhaler to loosen the cap with a ½ turn. Separate the Inhaler from the bottle and remove the cap by hand.
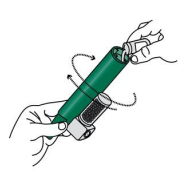
3. Tilt the PENTHROP Inhaler to a 45° angle and pour the total contents of one PENTHROP bottle into the base of the Inhaler whilst rotating.
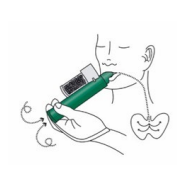
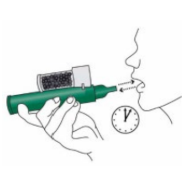
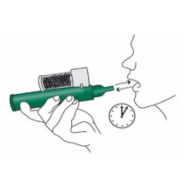
4. Place the wrist loop over the patient’s wrist. The patient inhales and exhales through the mouthpiece of the PENTHROP Inhaler to obtain analgesia. The first few breaths should be gentle. Thereafter the patient breathes normally through the Inhaler.
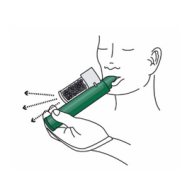
5. The patient exhales into the PENTHROP Inhaler. The exhaled vapour passes through the AC Chamber to adsorb any exhaled methoxyflurane.
6. If stronger analgesia is required, the patient can cover the dilutor hole on the AC Chamber with a finger during use.
7. If further pain relief is required, after the first bottle has been used use a second bottle if available. Alternatively use a second bottle from a new combination pack. Use in the same way as the first bottle in step 2 and 3. No need to remove the AC Chamber. Put used bottle into the plastic bag provided.
8. The patient should be instructed to inhale intermittently to achieve adequate analgesia. Continuous inhalation will reduce duration of use. Patients should be administered the minimum dose to achieve analgesia.
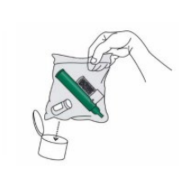
9. Replace cap onto PENTHROP bottle. Place used PENTHROP Inhaler and used bottle in sealed plastic bag and dispose of responsibly (see section 6.4).
4.9. Overdose
Adverse effects will include those for anaesthetic doses, see section 4.8 and section 4.4. In the event of overdose, anaesthetic effects may occur with signs of excessive drowsiness (including loss of consciousness), lowering blood pressure, respiratory depression, pallor and muscle relaxation. After PENTHROP discontinuation such overdose effects usually resolve quickly often with no other intervention required but cardiorespiratory supportive measures can be implemented if necessary. In the event of excessive urinary output following overdosage, fluid and electrolyte losses should be promptly replaced.
6.3. Shelf life
36 months.
6.4. Special precautions for storage
Store at or below 30°C in its original container.
Do not freeze.
Keep the product in the original container until required for use in order to protect from light. Keep the container tightly closed.
KEEP OUT OF REACH OF CHILDREN.
6.5. Nature and contents of container
3 ml of PENTHROP solution is filled into a 5 ml Type I amber glass screw neck bottle and closed with a white cap. PENTHROP is supplied in the following presentations:
- Combination pack containing one sealed bottle filled with 3 ml liquid, one PENTHROP inhaler and one Activated Carbon chamber in an outer carton box (pack of 1 or 10 units).
- Combination pack containing one sealed bottle filled with 3 ml liquid and one PENTHROP inhaler in an outer carton box (pack of 10 units).
6.6. Special precautions for disposal and other handling
After loading the PENTHROP Inhaler, replace cap onto PENTHROP bottle. After use, place used PENTHROP Inhaler and used bottle in a plastic bag, seal and dispose of responsibly.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.