PENTHROX Inhalation vapour Ref.[9285] Active ingredients:
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Medical Developments UK Limited, c/o Price Bailey LLP, Causeway House, 1 Dane Street, Bishops Stortford, Herts CM23 3BT, United Kingdom
Therapeutic indications
Emergency relief of moderate to severe pain in conscious adult patients with trauma and associated pain.
Posology and method of administration
PENTHROX should be self-administered under supervision of a person trained in its administration,
Posology
Adults
One bottle of 3 ml PENTHROX as a single dose, administered using the device provided. A second bottle should only be used where needed.
The frequency at which PENTHROX can be safely used is not established (see section 4.4). The following administration schedule is recommended: no more than 6 ml in a single day, administration on consecutive days is not recommended, and the total dose to a patient in a week should not exceed 15 ml.
Onset of pain relief is rapid and occurs after 6–10 inhalations. Patients should be instructed to inhale intermittently to achieve adequate analgesia. Patients are able to assess their own level of pain and titrate the amount of PENTHROX inhaled for adequate pain control. Continuous inhalation of a bottle containing 3 ml provides analgesic relief for up to 25-30 minutes. Intermittent inhalation may provide longer analgesic relief. Patients should be advised to use the lowest possible dose to achieve pain relief (see section 4.4).
Renal impairment
Methoxyflurane may cause renal failure if the recommended dose is exceeded. Caution should be exercised for patients diagnosed with clinical conditions that would pre-dispose to renal injury (see section 4.4).
Hepatic impairment
Cautious clinical judgement should be exercised when PENTHROX is to be used more frequently than on one occasion every 3 months (see section 4.4).
Paediatric population
PENTHROX should not be used in children and adolescents under 18 years.
Method of Administration
For inhalation use.
Instructions on the preparation of the PENTHROX Inhaler and correct administration are provided in the Figures below.
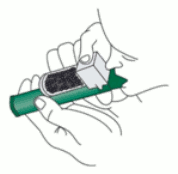
1. Ensure the Activated Carbon (AC) Chamber is inserted into the dilutor hole on the top of the PENTHROX Inhaler.
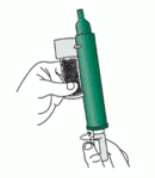
2. Remove the cap of the bottle by hand. Alternatively, use the base of the PENTHROX Inhaler to loosen the cap with a ½ turn. Separate the Inhaler from the bottle and remove the cap by hand.
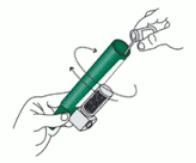
3. Tilt the PENTHROX Inhaler to a 45° angle and pour the total contents of one PENTHROX bottle into the base of the Inhaler whilst rotating.
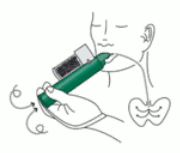
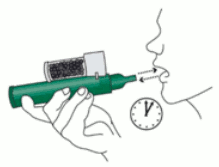
4. Place wrist loop over patient’s wrist. Patient inhales and exhales PENTHROX through the mouthpiece to obtain analgesia. First few breaths should be gentle and then breathe normally through Inhaler.
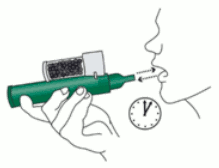
5. Patient exhales into the PENTHROX Inhaler. The exhaled vapour passes through the AC Chamber to adsorb any exhaled methoxyflurane.
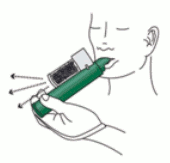
6. If stronger analgesia is required, patient can cover dilutor hole on the AC chamber with finger during use.
7. If further pain relief is required, after the first bottle has been used use a second bottle if available. Alternatively use a second bottle from a new combination pack. Use in the same way as the first bottle in step 2 and 3. No need to remove the AC Chamber. Put used bottle into the plastic bag provided.
8. Patient should be instructed to inhale intermittently to achieve adequate analgesia. Continuous inhalation will reduce duration of use. Minimum dose to achieve analgesia should be administered.
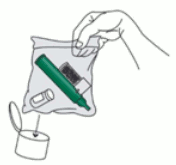
9. Replace cap onto PENTHROX bottle. Place used PENTHROX Inhaler and used bottle in sealed plastic bag and dispose of responsibly (see section 6.6).
The <physician,>
Overdose
Patients should be observed for signs of drowsiness, pallor and muscle relaxation following methoxyflurane administration.
High doses of methoxyflurane cause dose related nephrotoxicity. High output renal failure has occurred several hours or days after the administration of repeated high analgesic or anaesthetic doses of methoxyflurane.
Shelf life
36 months.
Special precautions for storage
This medicinal product does not require any special temperature storage conditions. For storage, PENTHROX combination pack should be kept in a locked cabinet, and should not be left on an open shelf.
Nature and contents of container
PENTHROX is supplied in the following presentations:
- One bottle with a tear off tamper-evident seal (packs of 10)
- Combination pack with one bottle of 3 ml PENTHROX, one PENTHROX Inhaler and one Activated Carbon (AC) chamber (packs of 1 or 10).
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
After loading the PENTHROX Inhaler, replace cap onto PENTHROX bottle. After use, place used PENTHROX Inhaler and used bottle in plastic bag provided, seal and dispose of responsibly.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.