PIFELTRO Film-coated tablet Ref.[7599] Active ingredients: Doravirine
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Co-administration with medicinal products that are strong cytochrome P450 CYP3A enzyme inducers is contraindicated as significant decreases in doravirine plasma concentrations are expected to occur, which may decrease the effectiveness of Pifeltro (see sections 4.4 and 4.5). These medicinal products include, but are not limited, to the following:
- carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- rifampicin, rifapentine
- St. John’s wort (Hypericum perforatum)
- mitotane
- enzalutamide
- lumacaftor
Special warnings and precautions for use
NNRTI substitutions and use of doravirine
Doravirine has not been evaluated in patients with previous virologic failure to any other antiretroviral therapy. NNRTI-associated mutations detected at screening were part of exclusion criteria in the Phase 2b/3-studies. A breakpoint for a reduction in susceptibility, yielded by various NNRTI substitutions, that is associated with a reduction in clinical efficacy has not been established (see section 5.1). There is not sufficient clinical evidence to support the use of doravirine in patients infected with HIV-1 with evidence of resistance to the NNRTI class.
Use with CYP3A inducers
Caution should be given to prescribing doravirine with medicinal products that may reduce the exposure of doravirine (see sections 4.3 and 4.5).
Immune reactivation syndrome
Immune reactivation syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, autoimmune hepatitis, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reactivation; however, the time to onset is more variable and can occur many months after initiation of treatment.
Lactose
The tablets contain lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Interaction with other medicinal products and other forms of interaction
Effects of other medicinal products on doravirine
Doravirine is primarily metabolised by CYP3A, and medicinal products that induce or inhibit CYP3A are expected to affect the clearance of doravirine (see section 5.2). Doravirine should not be co-administered with medicinal products that are strong CYP3A enzyme inducers as significant decreases in doravirine plasma concentrations are expected to occur, which may decrease the effectiveness of doravirine (see sections 4.3 and 5.2).
Co-administration with the moderate CYP3A inducer rifabutin decreased doravirine concentrations (see Table 1). When doravirine is co-administered with rifabutin, the doravirine dose should be increased to 100 mg twice daily (the doses should be taken approximately 12 hours apart) (see section 4.2).
Co-administration of doravirine with other moderate CYP3A inducers has not been evaluated, but decreased doravirine concentrations are expected. If co-administration with other moderate CYP3A inducers (e.g., dabrafenib, lesinurad, bosentan, thioridazine, nafcillin, modafinil, telotristat ethyl) cannot be avoided, the doravirine dose should be increased to 100 mg twice daily (the doses should be taken approximately 12 hours apart) (see section 4.2).
Co-administration of doravirine and medicinal products that are inhibitors of CYP3A may result in increased plasma concentrations of doravirine. However, no dose adjustment is needed when doravirine is co-administered with CYP3A inhibitors.
Effects of doravirine on other medicinal products
Doravirine at a dose of 100 mg once daily is not likely to have a clinically relevant effect on the plasma concentrations of medicinal products that are dependent on transport proteins for absorption and/or elimination or that are metabolized by CYP enzymes.
However, co-administration of doravirine and the sensitive CYP3A substrate midazolam resulted in a 18% decrease in midazolam exposure, suggesting that doravirine may be a weak CYP3A inducer. Therefore caution should be used when co-administering doravirine with medicinal products that are sensitive CYP3A substrates that also have a narrow therapeutic window (e.g. tacrolimus and sirolimus).
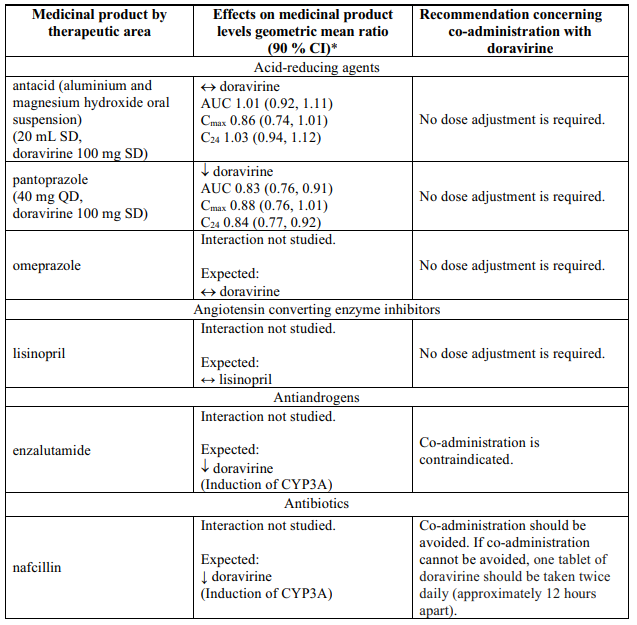
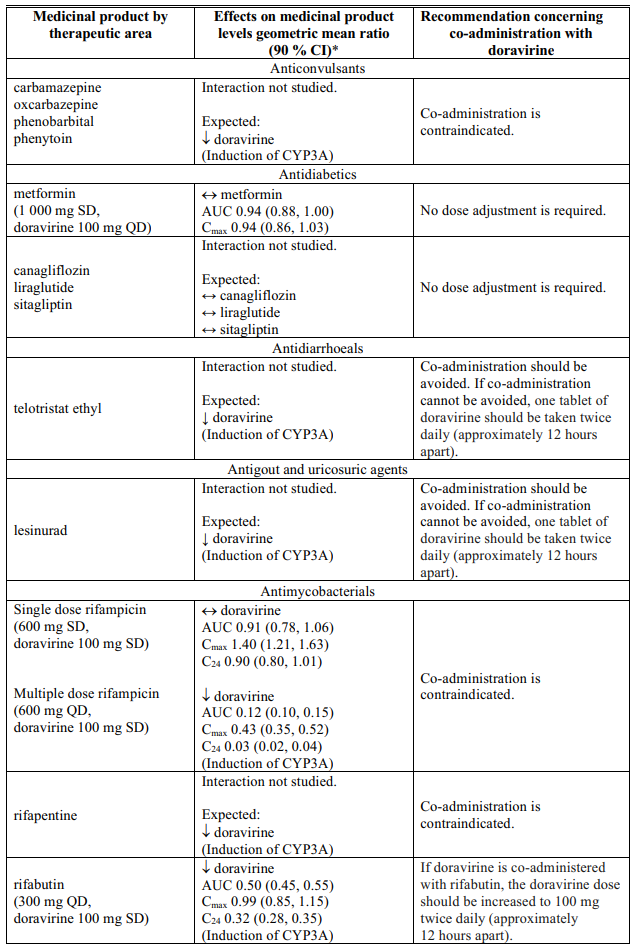
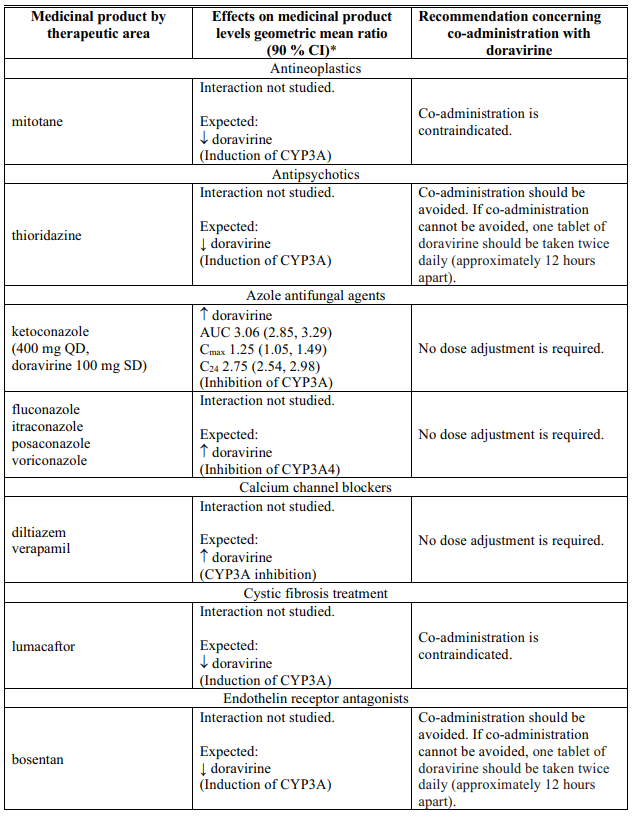
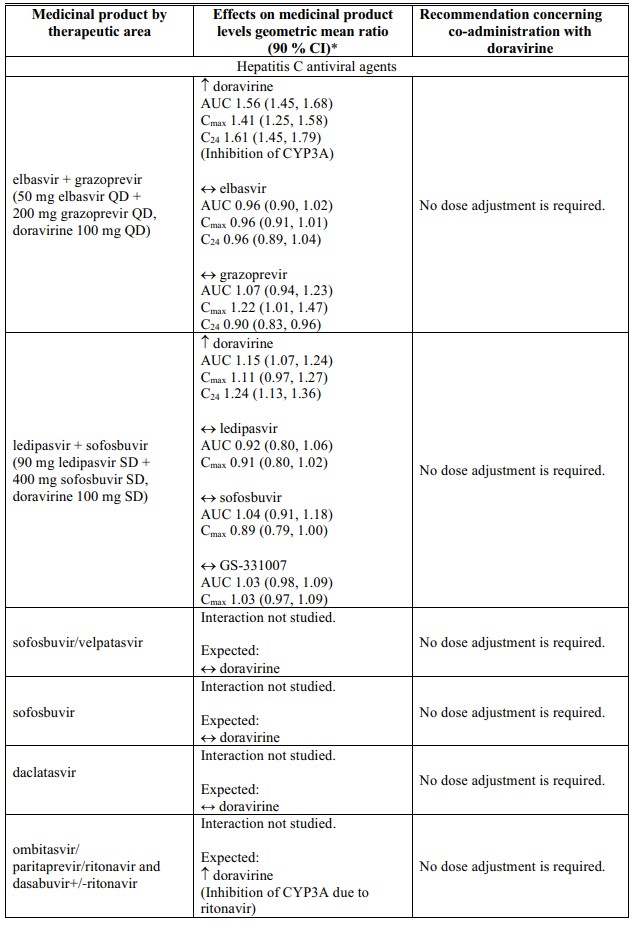
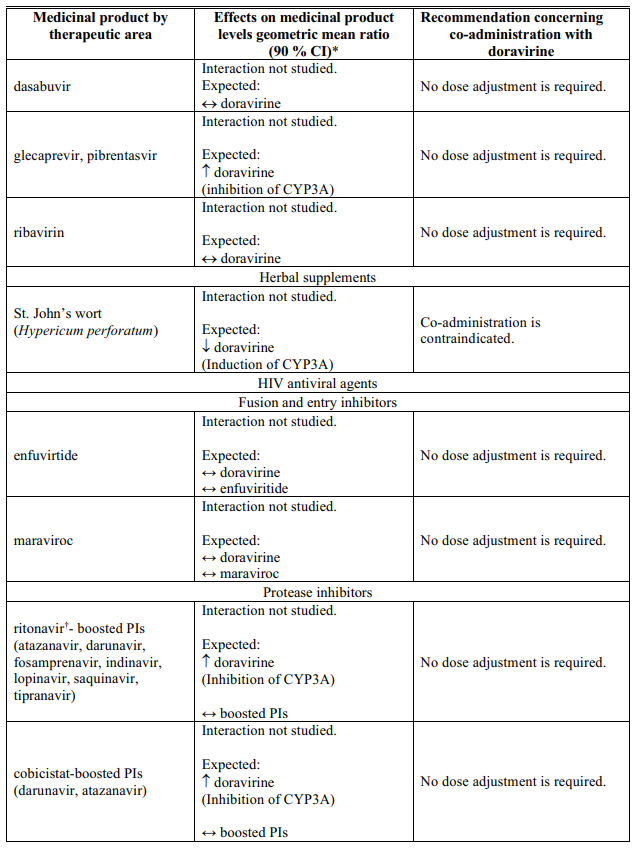
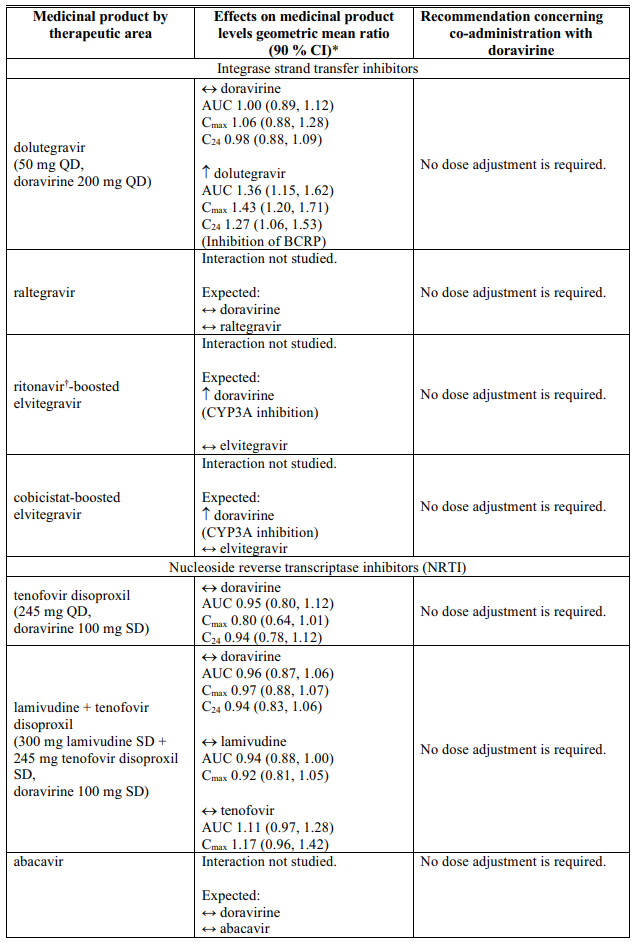
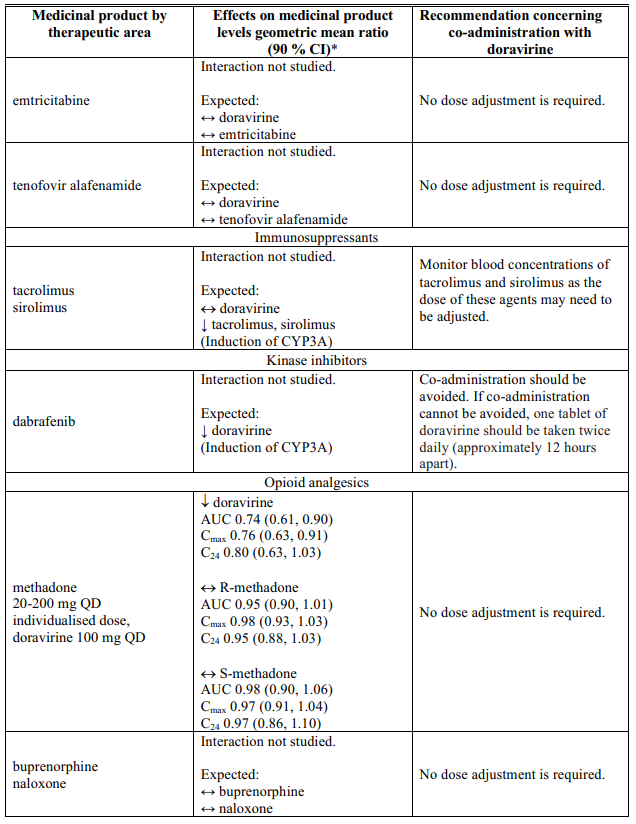
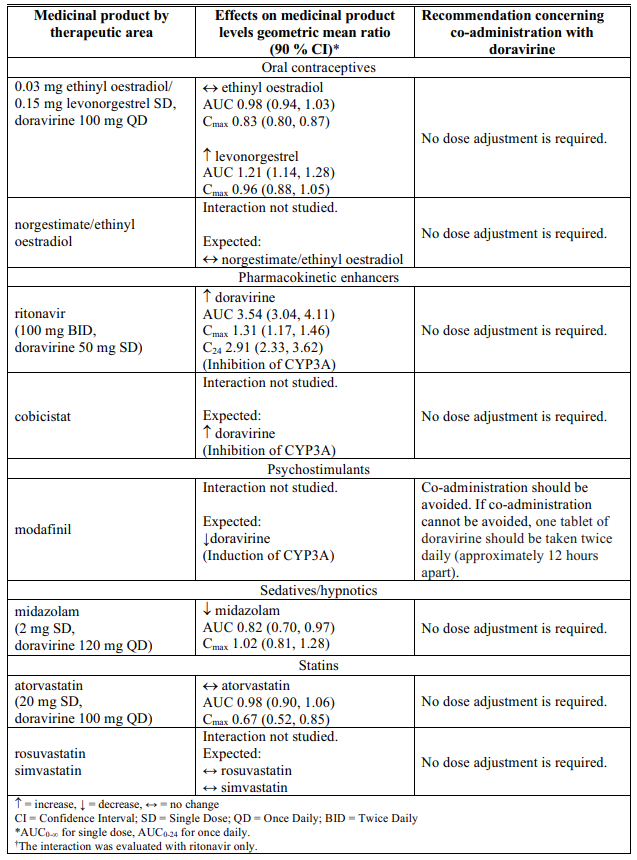
Interactions table
Table 1 shows the established and other potential medicinal product interactions with doravirine but is not all inclusive (increase is indicated as ↑, decrease is indicated as ↓, and no change as ↔).
Table 1. Interactions of doravirine with other medicinal products:
Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data from the use of doravirine in pregnant women.
Antiretroviral pregnancy registry: To monitor maternal-foetal outcomes in patients exposed to antiretroviral medicinal products while pregnant, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients in this registry.
Animal studies with doravirine do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
As a precautionary measure, it is preferable to avoid the use of doravirine during pregnancy.
Breast-feeding
It is unknown whether doravirine is excreted in human milk. Available pharmacodynamic/toxicological data in animals have shown excretion of doravirine in milk (see section 5.3).
It is recommended that women living with HIV do not breast-feed their infants in order to avoid transmission of HIV.
Fertility
No human data on the effect of doravirine on fertility are available. Animal studies do not indicate harmful effects of doravirine on fertility at exposure levels higher than the exposure in humans at the recommended clinical dose (see section 5.3).
Effects on ability to drive and use machines
Pifeltro has a minor influence on the ability to drive and use machines. Patients should be informed that fatigue, dizziness, and somnolence have been reported during treatment with doravirine (see section 4.8). This should be considered when assessing a patient’s ability to drive or operate machinery.
Undesirable effects
Summary of the safety profile
In phase 3 clinical trials with doravirine plus 2 NRTIs, the most frequently reported adverse reactions were nausea (4%) and headache (3%).
Tabulated summary of adverse reactions
The adverse reactions with doravirine plus 2 NRTIs from Phase 3 clinical trials (DRIVE FORWARD, DRIVE SHIFT and DRIVE AHEAD) are listed below by body system organ class and frequency. Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1 000 to <1/100) or rare (≥1/10 000 to <1/1 000).
Table 2. Tabulated summary of adverse reactions associated with doravirine used in combination with other antiretrovirals:
| Frequency | Adverse reactions |
|---|---|
| Infections and infestations | |
| Rare | rash pustular |
| Metabolism and nutrition disorders | |
| Uncommon | hypophosphataemia |
| Rare | hypomagnesaemia |
| Psychiatric disorders | |
| Common | abnormal dreams, insomnia1 |
| Uncommon | nightmare, depression2, anxiety3, irritability, confusional state, suicidal ideation |
| Rare | aggression, hallucination, adjustment disorder, mood altered, somnambulism |
| Nervous system disorders | |
| Common | headache, dizziness, somnolence |
| Uncommon | disturbance in attention, memory impairment, paraesthesia, hypertonia, poor quality sleep |
| Vascular disorders | |
| Uncommon | hypertension |
| Respiratory, thoracic and mediastinal disorders | |
| Rare | dyspnoea, tonsillar hypertrophy |
| Gastrointestinal disorders | |
| Common | nausea, diarrhoea, flatulence, abdominal pain4, vomiting |
| Uncommon | constipation, abdominal discomfort5, abdominal distension, dyspepsia, faeces soft6, gastrointestinal motility disorder7 |
| Rare | rectal tenesmus |
| Skin and subcutaneous tissue disorders | |
| Common | rash8 |
| Uncommon | pruritus |
| Rare | dermatitis allergic, rosacea |
| Musculoskeletal and connective tissue disorders | |
| Uncommon | myalgia, arthralgia |
| Rare | musculoskeletal pain |
| Renal and urinary disorders | |
| Rare | acute kidney injury, renal disorder, calculus urinary, nephrolithiasis |
| General disorders and administration site conditions | |
| Common | fatigue |
| Uncommon | asthenia, malaise |
| Rare | chest pain, chills, pain, thirst |
| Investigations | |
| Common | alanine aminotransferase increased9 |
| Uncommon | lipase increased, aspartate aminotransferase increased, amylase increased, haemoglobin decreased |
| Rare | blood creatine phosphokinase increased |
1 insomnia includes: insomnia, initial insomnia and sleep disorder
2 depression includes: depression, depressed mood, major depression, and persistent depressive disorder
3 anxiety includes: anxiety and generalised anxiety disorder
4 abdominal pain includes: abdominal pain, and abdominal pain upper
5 abdominal discomfort includes: abdominal discomfort, and epigastric discomfort
6 faeces soft includes: faeces soft and abnormal faeces
7 gastrointestinal motility disorder includes: gastrointestinal motility disorder, and frequent bowel movements
8 rash includes: rash, rash macular, rash erythematous, rash generalised, rash maculo-papular, rash papular, and urticarial
9 alanine aminotransferase increased includes: alanine aminotransferase increased andhepatocellular injury
Immune reactivation syndrome
In HIV-infected patients with severe immune deficiency at the time of initiation of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic infections may arise. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.4).
Paediatric population
The safety of doravirine as a component of doravirine/lamivudine/tenofovir disoproxil was evaluated in 45 HIV-1 infected virologically suppressed or treatment-naïve paediatric patients 12 to less than 18 years of age through Week 48 in an open-label trial (IMPAACT 2014 (Protocol 027)). The safety profile in paediatric subjects was similar to that in adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.