PIXUVRI Powder for concentrate for solution for infusion Ref.[9086] Active ingredients: Pixantrone
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Les Laboratoires Servier, 50, rue Carnot, 92284, Suresnes cedex, France
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, anthracyclines, and related substances.
ATC code: L01DB11
Mechanism of action
The active substance of Pixuvri is pixantrone, a cytotoxic aza-anthracenedione.
Unlike approved anthracyclines (doxorubicin and others) and anthracenediones (mitoxantrone), pixantrone is only a weak inhibitor of topoisomerase II. Moreover, unlike anthracyclines or anthracenediones, pixantrone directly alkylates DNA forming stable DNA adducts and cross-strand breaks. Furthermore, because it incorporates a nitrogen heteroatom into the ring structure and does not have ketone groups, pixantrone has less potential for generating reactive oxygen species, binding iron, and forming alcohol metabolites that are felt to cause the cardiac toxicity of anthracyclines. Due to this unique structure, pixantrone produced minimal cardiotoxicity in animal models compared with doxorubicin or mitoxantrone.
A comprehensive retrospective population PK/PD analysis of Phase 1 trials and combination regimens (Phase ½) demonstrated that progression-free survival and Grade 2-3 neutropenia were related to Pixuvri exposure.
Clinical efficacy and safety
The safety and efficacy of Pixuvri as single-agent therapy were evaluated in a multicentre, randomised, active controlled trial in patients with relapsed or refractory aggressive NHL after receiving at least two prior therapies. This study randomised 140 patients (1:1) to treatment with either Pixuvri or to an investigator chosen single-agent chemotherapy on the comparator arm. Patient demographics and baseline disease characteristics were well balanced between the treatment groups, and no statistically significant differences were noted. For the study overall, patient median age was 59, 61% were male, 64% were Caucasian, 76% had Ann Arbor stage III/IV disease at baseline, 74% had a baseline International Prognostic Index (IPI) score ≥2, and 60% had received ≥3 prior chemotherapies. Mantle cell lymphoma patients were not included in the pivotal study. Patients in PIX 301 were required to have been sensitive to prior anthracycline therapy (confirmed or unconfirmed CR or PR).
There is limited data in patients previously treated with rituximab (38 patients in the Pixuvri arm and 39 patients in the comparator arm). Tumour response was assessed by a blinded independent central review panel according to the international workshop to standardise response criteria for NHL. Patients treated with Pixuvri showed a significantly higher rate of complete responses and unconfirmed complete responses (CR/CRu), and a higher objective response rate (ORR), compared to the comparator group (see Table 4).
Table 4. Summary of response per independent assessment panel (ITT population):
| End-of-Treatment | End-of-Study | |||||
|---|---|---|---|---|---|---|
| Pixuvri (n=70) | Comparator (n=70) | P-value | Pixuvri (n=70) | Comparator (n=70) | P-value | |
| CR/CRu | 14 (20.0%) | 4 (5.7%) | 0.021 | 17 (24.3%) | 5 (7.1%) | 0.009 |
| CR | 8 (11.4%) | 0 (0%) | 11 (15.7%) | 0 (0.0%) | ||
| CRu | 6 (8.6%) | 4 (5.7%) | 6 (8.6%) | 5 (7.1%) | ||
| ORR (CR, Cru και PR) | 26 (37.1%) | 10 (14.3%) | 0.003 | 28 (40.0%) | 10 (14.3%) | 0.001 |
The Fisher exact test was used to compare proportions in the Pixuvri and comparator chemotherapeutic groups.
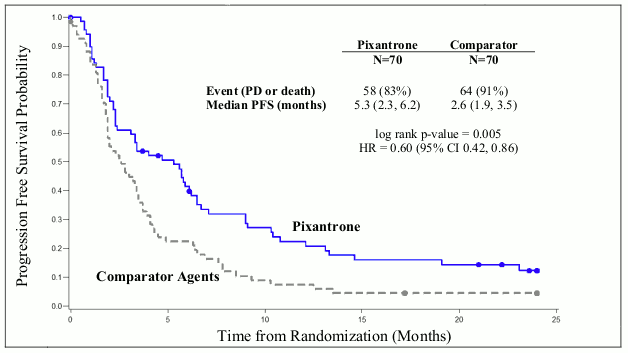
Patients treated with Pixuvri demonstrated 40% improvement in progression-free-survival compared to patients treated with comparator agents with 2.7 months longer median PFS (hazard ratio (HR)=0.60, logrank p=0.005) (see Figure 1 below).
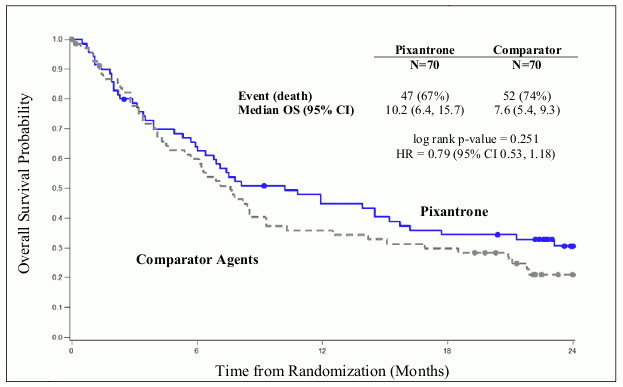
The median overall survival for patients treated with Pixuvri was 2.6 months longer compared to patients treated with comparator (HR=0.79, logrank p=0.25) (see Figure 2 below).
Figure 1. PIX301 Progression-free survival - end of study:
Figure 2. PIX 301 Overall survival–end of study:
The results in the rituximab pretreated patients still showed superior treatment benefit with Pixuvri over the comparator for overall response rate (31.6% with Pixuvri versus 17.9% with the comparator) and median progression-free survival (3.3 months with Pixuvri versus 2.5 months with the comparator). However, the benefit of Pixuvri has not been established when used as fifth line or greater in patients refractory to last therapy, and there is very limited data in this group of patients.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Pixuvri in all subsets of the paediatric population in treatment of non-Hodgkin lymphoma. (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Following intravenous administration, plasma concentrations of pixantrone reached the maximal concentration at the end of infusion and then declined poly-exponentially. The pharmacokinetics of Pixuvri was dose-independent in the 3 mg/m² to105 mg/m² dose range and no substantial differences were observed when the medicinal product was given as a single agent or in combination studies. Average exposures as single agent accounted for:
| Pixuvri Dose (mg/m²) | Number of patients | AUC (0-24h) (ng.hr/ml) |
|---|---|---|
| 33 | 3 | 982 ± 115 |
| 49 | 6 | 1727 ± 474 |
| 88 | 2 | 3811 |
From an analysis of population PK data, for a target recorded dose of 50 mg/m² of pixantrone the median 28-day cycle exposure was 6320 ng.hr/ml (90% CI, 5990-6800 ng.hr/ml), for 3 doses/4 week cycle.
Distribution
Pixuvri has a large volume of distribution of 25.8 l and is approximately 50% bound to plasma proteins.
Biotransformation
Acetylated metabolites are the major biotransformation products of pixantrone. However, in vitro, conversion of pixantrone into the acetylated metabolites by either NAT1 or NAT2 was very limited. In human urine, the compound was mainly excreted unchanged, and very small amounts of phase I and phase II acetylated metabolites were found. Therefore, metabolism does not appear to be an important elimination pathway for pixantrone. Acetylated metabolites were pharmacologically inactive and metabolically stable.
Elimination
Pixantrone has a moderate to high total plasma clearance of 72.7 l/hr and a low renal excretion accounting for less than 10% of the administered dose in 0-24 hours. The terminal half-life ranged from 14.5 to 44.8 hr with a mean of 23.3 ± 8.0 (n=14, CV=34%) and a median of 21.2 hr. Due to the limited contribution of renal clearance, plasma clearance is mainly non-renal. Pixuvri may be metabolised in the liver and/or excreted in the bile. As metabolism appears to be limited, biliary excretion of unchanged pixantrone may be the major elimination pathway. Hepatic clearance approximates the hepatic plasma flow, suggesting a high hepatic extraction ratio and, therefore, efficient parent active substance elimination. Hepatic uptake of pixantrone is possibly mediated by OCT1 active transporters and biliary excretion by P-gp and BCRP.
Pixantrone had only a weak or no capability to inhibit P-gp, BCRP, and BSEP transport mechanism in vitro.
Pixantrone did inhibit OCT1-mediated metformin transport in vitro, but is not expected to inhibit OTC1 in vivo at clinically relevant concentrations.
Pixantrone was a poor inhibitor of OATP1B1 and OATP1B3 uptake transporters in vitro.
Linearity/non-linearity
Pharmacokinetics of pixantrone proved to be linear in a broad range of doses, from 3 mg/m² to 105 mg/m².
Pharmacokinetic/pharmacodynamic relationship(s)
A relationship between plasma exposure to pixantrone and neutrophil count has been observed.
Preclinical safety data
After a single intravenous administration of Pixuvri at 29 mg/kg and 38 mg/kg, immediate deaths were seen in mice (114 mg/m², LD10). Decreases in white and red blood cells and alterations in bone marrow, spleen, kidney, and testes were observed. Similar findings were reported in rats, and in dogs at 116 mg/m². In dogs, tachycardia and electrocardiography (ECG) changes occurred immediately after treatment.
In repeated-dose studies in mice, rats, and dogs, the main findings were myelotoxicity, nephrotoxicity (except dogs), and testicular damage.
In dogs, Pixuvri given at 0.5 to 0.9 mg/kg for six cycles did not cause mortality or severe clinical signs, including ECG or body weight changes. Males were more sensitive to treatment, with respect to reduction in white blood cells and platelet count (reversible) and lymphoid depletion (spleen and thymus), as well as the marked toxicity to reproductive organs, as expected from a cytotoxic agent. Except for a transient increase in exposure in females after the third cycle, there were no marked differences in pharmacokinetic parameters. Males showed, however, slightly higher exposure than females.
In dogs, the heart was not affected by treatment, as no ECG changes were seen at different treatment times, nor heart changes were detected at gross- and histopathology. Kidney function and histology were similarly not affected both in 4- and 26-week studies.
The cardiotoxic potential of Pixuvri compared with equiactive doses of doxorubicin and mitoxantrone in treatment-naïve and doxorubicin-pre-treated mice was evaluated. Pixantrone dimaleate up to 27 mg/kg given twice a week for 4 weeks did not induce any cardiotoxic effects, while mitoxantrone, as expected, was cardiotoxic at all tested doses (0.6, 1.6, and 1.5 mg/kg). Slight nephropathy was induced by Pixuvri. Minimal cardiotoxicity of Pixuvri was also demonstrated with repeat treatment cycles at the same doses.
Genotoxicity studies confirmed the potential for clastogenic effects in mammalian cells in vitro and in vivo. Pixuvri was mutagenic in the Ames test, increased the number of chromosomal aberrations in human lymphocytes, and increased the frequency of micronuclei in vivo.
Pixuvri caused maternal and foetal toxicity in rats and rabbits, even at a dose as low as 1.8 mg/kg given on days 9-11 of pregnancy, higher doses resulting in abortions and total embryo resorption. Embryotoxicity was characterised by reduced mean foetal weight, foetal malformations and incomplete or delayed foetal ossification. No long-term animal studies have been performed to establish the carcinogenic potential of Pixuvri. No local tolerance study was conducted.
Pixuvri has been shown to cause phototoxic effects on 3T3 cells in vitro.
In a colony-forming units study in mice, the myelotoxicity of Pixuvri and mitoxantrone administered at their LD10 (pixantrone dimaleate 38 mg/kg and mitoxantrone 6.1 mg/kg) was similar.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.