PLAQUENIL Film coated tablet Ref.[50772] Active ingredients: Hydroxychloroquine
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2022 Publisher: Pharmacy Retailing (NZ) Ltd t/a Healthcare Logistics, PO Box 62027, Sylvia Park Auckland 1644, Freecall: 0800 283 684, Email: medinfo.australia@sanofi.com
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antimalarials
ATC code: P01BA02
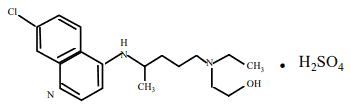
Hydroxychloroquine sulfate is designated chemically as 2-{N-(4-(7-Chloro-4- quinolylamino)pentyl)- -N-ethylamino}ethanol sulfate, and has the following chemical structure:
C18H26ClN3O, H2SO4 Molecular Weight: 433.96
CAS No. 747-36-4 (hydroxychloroquine sulfate), CAS No. 118-42-3 (hydroxychloroquine).
Mechanism of action
Anti-malarial. Plaquenil also exerts a beneficial effect in mild systemic and discoid lupus erythematosus and rheumatoid arthritis. The precise mechanism of action is not known.
Malaria
Like chloroquine phosphate, Plaquenil is highly active against the erythrocytic forms of P.vivax and P.malariae and most strains of P.falciparum (but not the gametocytes of P.falciparum).
Plaquenil does not prevent relapses in patients with vivax or malariae malaria because it is not effective against exo-erythrocytic forms of the parasite, nor will it prevent vivax or malariae infection when administered as a prophylactic. It is highly effective as a suppressive agent in patients with vivax or malariae malaria, in terminating acute attacks, and significantly lengthening the interval between treatment and relapse. In patients with falciparum malaria it abolishes the acute attack and effects complete cure of the infection, unless due to a resistant strain of P.falciparum.
5.2. Pharmacokinetic properties
Hydroxychloroquine has actions, pharmacokinetics and metabolism similar to those of chloroquine. Following oral administration, hydroxychloroquine is rapidly and almost completely absorbed. In one study, mean peak plasma hydroxychloroquine concentrations following a single dose of 400mg in healthy subjects ranged from 53-208ng/mL with a mean of 105ng/mL. The mean time to peak plasma concentration was 1.83 hours. The mean plasma elimination half-life varied, depending on the post-administration period, as follows: 5.9 hours (at Cmax -10 hours), 26.1 hours (at 10-48 hours) and 299 hours (at 48-504 hours). The parent compound and metabolites are widely distributed in the body and elimination is mainly via the urine, where 3% of the administered dose was recovered over 24 hours in one study.
Absorption
Following oral administration, peak blood concentration is achieved in approximately 4 hours. Absolute oral bioavailability is 79%.
Distribution
Hydroxychloroquine has a large volume of distribution due to extensive tissue accumulation (5500 L in, 44 000 L in plasma), and has been shown to accumulate in blood cells, with a blood to plasma ratio of 7.2. Approximately 50% of hydroxychloroquine is bound to plasma proteins.
Metabolism
Hydroxychloroquine is mainly metabolized to N-desethylhydroxychloroquine, and two other metabolites in common with chloroquine, desethylchloroquine and bidesethylchloroquine. It can be extrapolated from chloroquine, that hydroxychloroquine could be metabolized in vitro by the same CYPs as for chloroquine, i.e. CYP2C8 and CYP3A, and to a lesser extent by CYP2D6.
Elimination
Hydroxychloroquine presents a multi-phasic elimination profile with a long terminal half-life ranging from 30 to 60 days. Approximately 20-25% of the hydroxychloroquine dose is eliminated as unchanged drug in the urine. Preclinical safety data
There are limited data on hydroxychloroquine genotoxicity. Chloroquine is reported in the literature to elicit both gene mutations and chromosomal/DNA breaks in some in vitro systems but not others and in in vivo studies using rodents when dosed via the intraperitoneal route. Chromosomal effects were not observed in vivo when chloroquine was administered orally.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.