PLAVIX Film-coated tablet Ref.[10598] Active ingredients: Clopidogrel
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
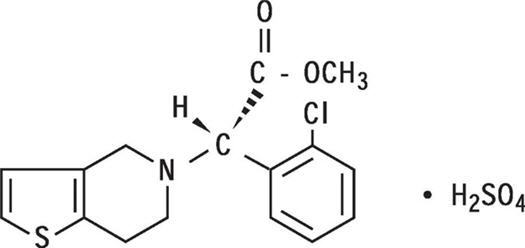
Plavix (clopidogrel tablets) is a thienopyridine class inhibitor of P2Y12 ADP platelet receptors. Chemically it is methyl (+)-α(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C16H16ClNO2S•H2SO4 and its molecular weight is 419.9.
The structural formula is as follows:
Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but freely soluble at pH 1. It also dissolves freely in methanol, dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether. It has a specific optical rotation of about +56°.
Plavix for oral administration is provided as either pink, round, biconvex, debossed, film-coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base or pink, oblong, debossed, film-coated tablets containing 391.5 mg of clopidogrel bisulfate which is the molar equivalent of 300 mg of clopidogrel base.
Each tablet contains hydrogenated castor oil, hydroxypropyl cellulose, mannitol, microcrystalline cellulose, and polyethylene glycol 6000 as inactive ingredients. The pink film coating contains ferric oxide, hypromellose 2910, lactose monohydrate, titanium dioxide, and triacetin. The tablets are polished with Carnauba wax.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
Plavix (clopidogrel tablets) 75 mg are available as pink, round, biconvex, film-coated tablets debossed with “75” on one side and “1171” on the other. Tablets are provided as follows:
Plavix (clopidogrel tablets) 300 mg are available as pink, oblong, film-coated tablets debossed with “300” on one side and “1332” on the other. Tablets are provided as follows:
Distributed by: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Bridgewater, NJ 08807 |
Drugs
| Drug | Countries | |
|---|---|---|
| PLAVIX | Austria, Brazil, Canada, Cyprus, Germany, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Mexico, Netherlands, Poland, Romania, Singapore, Tunisia, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.