POMALYST Capsule Ref.[10916] Active ingredients: Pomalidomide
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Pomalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Cellular activities of pomalidomide are mediated through its target cereblon, a component of a cullin ring E3 ubiquitin ligase enzyme complex. In vitro, in the presence of drug, substrate proteins (including Aiolos and Ikaros) are targeted for ubiquitination and subsequent degradation leading to direct cytotoxic and immunomodulatory effects. In in vitro cellular assays, pomalidomide inhibited proliferation and induced apoptosis of hematopoietic tumor cells. Additionally, pomalidomide inhibited the proliferation of lenalidomide-resistant multiple myeloma (MM) cell lines and synergized with dexamethasone in both lenalidomide-sensitive and lenalidomide-resistant cell lines to induce tumor cell apoptosis. Pomalidomide enhanced T cell- and natural killer (NK) cell-mediated immunity and inhibited production of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes. Pomalidomide demonstrated anti-angiogenic activity in a mouse tumor model and in the in vitro umbilical cord model.
12.2. Pharmacodynamics
Pomalidomide exposure-response analyses showed that there was no relationship between systemic pomalidomide exposure level and efficacy or safety following pomalidomide dose of 4 mg.
Cardiac Electrophysiology
The QTc prolongation potential of pomalidomide was evaluated in a single center, randomized, double-blind crossover study (N=72) using 4 mg pomalidomide, 20 mg pomalidomide, placebo, and 400 mg moxifloxacin (positive control). No significant QTc prolongation effect of pomalidomide was observed following pomalidomide doses of 4 and 20 mg.
12.3. Pharmacokinetics
In patients with MM who received POMALYST 4 mg daily alone or in combination with dexamethasone, pomalidomide steady-state drug exposure was characterized by AUC (CV%) of 860 (37%) ng∙h/mL and Cmax (CV%) of 75 (32%) ng/mL. In patients with Kaposi sarcoma (KS) who received POMALYST 5 mg daily, pomalidomide steady-state drug exposure was characterized by AUC of 462.3 ng∙h/mL (82%) and Cmax of 53.1 ng/mL (50%).
Absorption
Following administration of single oral doses of POMALYST, the maximum plasma concentration (Cmax) for pomalidomide occurs at 2 to 3 hours postdose in patients with MM or KS.
Effect of Food
Co-administration of POMALYST with a high-fat meal (approximately 50% of the total caloric content) and high-calorie meal (approximately 800 to 1000 calories) (the meal contained approximately 150, 250, and 500 to 600 calories from protein, carbohydrates, and fat, respectively) delays the Tmax by 2.5 hours, decreased mean plasma Cmax and AUC in healthy subjects by about 27% and 8%, respectively.
Distribution
Pomalidomide has a mean apparent volume of distribution (Vd/F) between 62 and 138 L at steady state in patients with MM or KS.
Pomalidomide is distributed in semen of healthy subjects at a concentration of approximately 67% of plasma level at 4 hours postdose (~Tmax) after 4 days of 2 mg once-daily dosing.
Human plasma protein binding of pomalidomide ranges from 12% to 44% and is not concentration dependent. Pomalidomide is a substrate for P-gp.
Elimination
Pomalidomide has a mean total body clearance (CL/F) of 7-10 L/h in patients with MM or KS. Pomalidomide is eliminated with a median plasma half-life of 9.5 hours in healthy subjects and 7.5 hours in patients with MM or KS.
Metabolism
Pomalidomide is primarily metabolized in the liver by CYP1A2 and CYP3A4. Minor contributions from CYP2C19 and CYP2D6 were also observed in vitro.
Excretion
Following a single oral administration of [14C]-pomalidomide to healthy subjects, approximately 73% and 15% of the radioactive dose was eliminated in urine and feces, respectively, with approximately 2% and 8% of the radiolabeled dose eliminated unchanged as pomalidomide in urine and feces.
Specific Populations
Age (61 to 85 years old), sex and race have no clinically significant effect on the systemic exposure of pomalidomide.
Patients with Renal Impairment
Pomalidomide pharmacokinetic parameters were not significantly affected in patients with moderate (30 mL/min ≤ CLcr < 60 mL/min) or severe (15 mL/min ≤ CLcr < 30 mL/min) renal impairment relative to patients with normal renal function (CLcr ≥ 60 mL/min). Mean exposure (AUC) to pomalidomide increased by 38% in patients with severe renal impairment requiring dialysis (CLcr< 30 mL/min requiring dialysis) and 40% in patients with end stage renal disease (CLcr< 15 mL/min) on non-dialysis days. In patients with severe renal impairment requiring dialysis, the estimated dialysis clearance is approximately 12 L/h which is higher than pomalidomide total body clearance, indicating hemodialysis will remove pomalidomide from the blood circulation.
Patients with Hepatic Impairment
Mean exposure (AUC) of pomalidomide increased by 51%, 58% and 72% in subjects with mild, moderate or severe hepatic impairment as defined by Child-Pugh criteria, respectively.
Drug Interaction Studies
Clinical Studies
Co-administration of POMALYST with the following drugs did not increase pomalidomide exposure to a clinically significant extent: ketoconazole (a strong CYP3A4 and P-gp inhibitor), carbamazepine (a strong CYP3A4 inducer) and dexamethasone (a weak to moderate CYP3A4 inducer). Co-administration of POMALYST with drugs that are CYP1A2 inducers has not been studied.
CYP1A2 Inhibitors: Co-administration of fluvoxamine (a strong CYP1A2 inhibitor) with POMALYST increased mean [90% confidence interval] pomalidomide exposure by 125% [98% to 157%] compared to POMALYST alone in healthy subjects. Co-administration of fluvoxamine in the presence of ketoconazole (a strong CYP3A4 and P-gp inhibitor) with POMALYST increased mean pomalidomide exposure by 146% [126% to 167%] compared to POMALYST administered alone in healthy subjects, indicating the predominant effect of CYP1A2 inhibition in the increase of pomalidomide exposure [see Dosage and Administration (2.6) and Drug Interactions (7.1)].
Strong CYP3A4 and P-gp Inhibitors: Co-administration of ketoconazole (a strong CYP3A4 and P-gp inhibitor) in 16 healthy male subjects increased AUC of pomalidomide by 19% compared to POMALYST administered alone.
Strong CYP1A2 Inducers: Co-administration of POMALYST with drugs that are CYP1A2 inducers has not been studied and may reduce pomalidomide exposure.
Strong CYP3A4 Inducers: Co-administration of carbamazepine to 16 healthy male subjects decreased AUC of pomalidomide by 20% with a 90% confidence interval [13% to 27%] compared to when pomalidomide was administered alone.
Dexamethasone: Co-administration of multiple doses of 4 mg POMALYST with 20 mg to 40 mg dexamethasone (a weak to moderate inducer of CYP3A4) to patients with MM had no effect on the pharmacokinetics of pomalidomide compared to when pomalidomide was administered alone.
Smoking: In 14 healthy male subjects who smoked 25 cigarettes per day for a total of 10 days, after single oral dose of 4 mg POMALYST, Cmax of pomalidomide increased 14% while AUC of pomalidomide decreased 32%, compared to that in 13 healthy male subjects who were non-smokers.
In Vitro Studies
Pomalidomide does not inhibit or induce cytochrome p450 enzymes or transporters in vitro.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies examining the carcinogenic potential of pomalidomide have not been conducted. One of 12 monkeys dosed with 1 mg/kg of pomalidomide (an exposure approximately 15-fold of the exposure in patients at the recommended dose of 4 mg/day) developed acute myeloid leukemia in a 9-month repeat-dose toxicology study.
Pomalidomide was not mutagenic or clastogenic in a battery of tests, including the bacteria reverse mutation assay (Ames test), the in vitro assay using human peripheral blood lymphocytes, and the micronucleus test in orally treated rats administered doses up to 2000 mg/kg/day.
In a fertility and early embryonic development study in rats, drug-treated males were mated with untreated or treated females. Pomalidomide was administered to males and females at doses of 25 to 1000 mg/kg/day. When treated males were mated with treated females, there was an increase in post-implantation loss and a decrease in mean number of viable embryos at all dose levels. There were no other effects on reproductive functions or the number of pregnancies. The lowest dose tested in animals resulted in an exposure (AUC) approximately 100-fold of the exposure in patients at the recommended dose of 4 mg/day. When treated males in this study were mated with untreated females, all uterine parameters were comparable to the controls. Based on these results, the observed effects were attributed to the treatment of females.
14. Clinical Studies
14.1 Multiple Myeloma
Trial 1
Trial 1 was a phase 2, multicenter, randomized open-label study in patients with relapsed multiple myeloma (MM) who were refractory to their last myeloma therapy and had received lenalidomide and bortezomib. Patients were considered relapsed if they had achieved at least stable disease for at least 1 cycle of treatment to at least 1 prior regimen and then developed progressive disease. Patients were considered refractory if they experienced disease progression on or within 60 days of their last therapy. A total of 221 patients were randomized to receive POMALYST alone or POMALYST with Low-dose Dex. In Trial 1, the safety and efficacy of POMALYST 4 mg, once daily for 21 of 28 days, until disease progression, were evaluated alone and in combination with Low-dose Dex (40 mg/day given only on Days 1, 8, 15, and 22 of each 28-day cycle for patients aged 75 years or younger, or 20 mg/day given only on Days 1, 8, 15, and 22 of each 28-day cycle for patients aged greater than 75 years). Patients in the POMALYST alone arm were allowed to add Low-dose Dex upon disease progression.
Table 7 summarizes the baseline patient and disease characteristics in Trial 1. The baseline demographics and disease characteristics were balanced and comparable between the study arms.
Table 7. Baseline Demographic and Disease-Related Characteristics – Trial 1:
| POMALYST (n=108) | POMALYST + Low-dose Dex (n=113) | |
|---|---|---|
| Patient Characteristics | ||
| Median age, years (range) | 61 (37-88) | 64 (34-88) |
| Age distribution, n (%) | ||
| <65 years | 65 (60.2) | 60 (53.1) |
| ≥65 years | 43 (39.8) | 53 (46.9) |
| Sex, n (%) | ||
| Male | 57 (52.8) | 62 (54.9) |
| Female | 51 (47.2) | 51 (45.1) |
| Race/ethnicity, n (%) | ||
| White | 86 (79.6) | 92 (81.4) |
| Black or African American | 16 (14.8) | 17 (15) |
| All other race | 6 (5.6) | 4 (3.6) |
| ECOG Performance, n (%) | ||
| Status 0-1 | 95 (87.9) | 100 (88.5) |
| Disease Characteristics | ||
| Number of prior therapies Median (min, max) | 5 (2, 12) | 5 (2, 13) |
| Prior transplant, n (%) | 82 (75.9) | 84 (74.3) |
| Refractory to bortezomib and lenalidomide, n (%) | 64 (59.3) | 69 (61.1) |
Data cutoff: 01 April 2011
Table 8 summarizes the analysis results of overall response rate (ORR) and duration of response (DOR), based on assessments by the Independent Review Adjudication Committee for the treatment arms in Trial 1. ORR did not differ based on type of prior antimyeloma therapy.
Table 8. Trial 1 Results:
| POMALYST* (n=108) | POMALYST + Low-dose Dex (n=113) | |
|---|---|---|
| Response | ||
| Overall Response Rate (ORR)†, n (%) | 8 (7.4) | 33 (29.2) |
| 95% CI for ORR (%) | (3.3, 14.1) | (21.0, 38.5) |
| Complete Response (CR), n (%) | 0 (0.0) | 1 (0.9) |
| Partial Response (PR), n (%) | 8 (7.4) | 32 (28.3) |
| Duration of Response (DOR) | ||
| Median, months | NE | 7.4 |
| 95% CI for DOR (months) | NE | (5.1, 9.2) |
CI, confidence interval; NE, not established (the median has not yet been reached).
Data cutoff: 01 April 2011
* Results are prior to the addition of dexamethasone.
† ORR = PR + CR per EBMT criteria.
Trial 2
Trial 2 was a Phase 3 multi-center, randomized, open-label study, where POMALYST + Low-dose Dex therapy was compared to High-dose Dex in adult patients with relapsed and refractory MM, who had received at least two prior treatment regimens, including lenalidomide and bortezomib, and demonstrated disease progression on or within 60 days of the last therapy. Patients with creatinine clearance ≥45mL/min qualified for the trial. A total of 455 patients were enrolled in the trial: 302 in the POMALYST + Low-dose Dex arm and 153 in the High-dose Dex arm. Patients in the POMALYST + Low-dose Dex arm were administered 4 mg POMALYST orally on Days 1 to 21 of each 28-day cycle. Dexamethasone (40 mg) was administered once per day on Days 1, 8, 15 and 22 of a 28-day cycle. Patients >75 years of age started treatment with 20 mg dexamethasone using the same schedule. For the High-dose Dex arm, dexamethasone (40 mg) was administered once per day on Days 1 through 4, 9 through 12, and 17 through 20 of a 28-day cycle. Patients >75 years of age started treatment with 20 mg dexamethasone using the same schedule. Treatment continued until patients had disease progression.
Baseline patient and disease characteristics were balanced and comparable between the study arms, as summarized in Table 9. Overall, 94% of patients had disease refractory to lenalidomide, 79% had disease refractory to bortezomib and 74% had disease refractory to both lenalidomide and bortezomib.
Table 9. Baseline Demographic and Disease-Related Characteristics – Trial 2:
| POMALYST + Low-dose Dex | High-dose Dex | |||
|---|---|---|---|---|
| (N=302) | (N=153) | |||
| Patient Characteristics | ||||
| Median Age, years (range) | 64 (35, 84) | 65 (35, 87) | ||
| Age Distribution n (%) | ||||
| <65 years | 158 (52) | 74 (48) | ||
| ≥65 years | 144 (48) | 79 (52) | ||
| Sex n (%) | ||||
| Male | 181 (60) | 87 (57) | ||
| Female | 121 (40) | 66 (43) | ||
| Race/Ethnicity n (%) | ||||
| White | 244 (81) | 113 (74) | ||
| Black or African American | 4 (1) | 3 (2) | ||
| Asian | 4 (1) | 0 (0) | ||
| Other Race | 2 (1) | 2 (1) | ||
| Not Collected | 48 (16) | 35 (23) | ||
| ECOG Performance n (%) | ||||
| Status 0 | 110 (36) | 36 (24) | ||
| Status 1 | 138 (46) | 86 (56) | ||
| Status 2 | 52 (17) | 25 (16) | ||
| Status 3 | 0 (0) | 3 (2) | ||
| Missing | 2 (1) | 3 (2) | ||
| Disease Characteristics | ||||
| Number of Prior Therapies Median, (Min, Max) | 5 (2, 14) | 5 (2, 17) | ||
| Prior stem cell transplant n (%) | 214 (71) | 105 (69) | ||
| Refractory to bortezomib and lenalidomide n (%) | 225 (75) | 113 (74) | ||
Data cutoff: 01March 2013
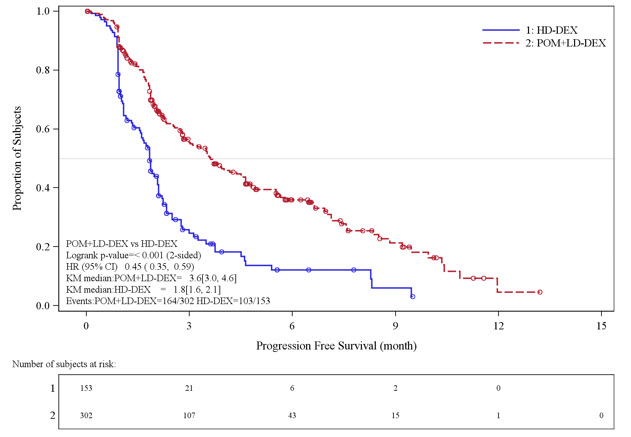
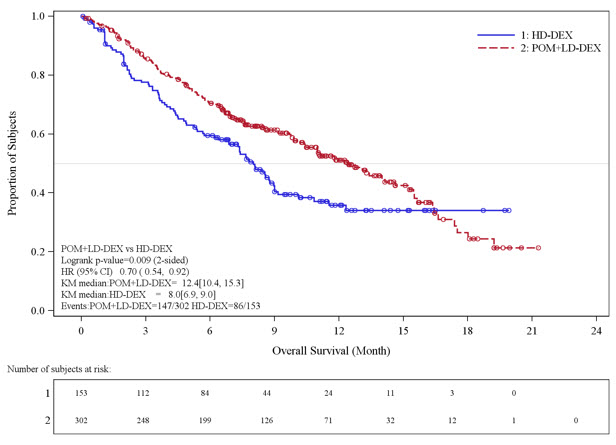
Table 10 summarizes the progression free survival (PFS) and overall response rate (ORR) based on the assessment by the Independent Review Adjudication Committee (IRAC) review at the final PFS analysis and overall survival (OS) at the OS analysis. PFS was significantly longer with POMALYST + Low-dose Dex than High-dose Dex: HR 0.45 (95% CI: 0.35-0.59 p<0.001). OS was also significantly longer with POMALYST + Low-dose Dex than High-dose Dex: HR 0.70 (95% CI: 0.54-0.92 p=0.009). The Kaplan-Meier curves for PFS and OS for the ITT population are provided in Figures 1 and 2, respectively.
Table 10. Trial 2 Results:
| POMALYST + Low-dose Dex | High-dose Dex | |
|---|---|---|
| (N=302) | (N=153) | |
| Progression Free Survival Time | ||
| Number (%) of events | 164 (54.3) | 103 (67.3) |
| Median* (2-sided 95% CI) (months) | 3.6 [3.0, 4.6] | 1.8 [1.6, 2.1] |
| Hazard Ratio (Pom+LD-Dex:HD-Dex) 2-Sided 95% CI† | 0.45 [0.35, 0.59] | |
| Log-Rank Test 2-sided P-Value‡ | <0.001 | |
| Overall Survival Time§ | ||
| Number (%) of deaths | 147 (48.7) | 86 (56.2) |
| Median* (2-sided 95% CI) (months) | 12.4 [10.4, 15.3] | 8.0[6.9, 9.0] |
| Hazard Ratio (Pom+LD-Dex:HD-Dex) 2-Sided 95% CI¶ | 0.70 [0.54, 0.92] | |
| Log-Rank Test 2-sided P-Value#,Þ | 0.009 | |
| Overall Response Rate, n (%) | 71 (23.5) | 6 (3.9) |
| Complete Response | 1 (0.3) | 0 |
| Very Good Partial Response | 8 (2.6) | 1 (0.7) |
| Partial Response | 62 (20.5) | 5 (3.3) |
Note: CI=Confidence interval; HD-Dex=High dose dexamethasone; IRAC=Independent Review Adjudication Committee; LD-Dex=Low dose dexamethasone.
Data cutoff: 07 Sep 2012 for PFS
Data cutoff: 01 Mar 2013 for OS and ORR
* The median is based on Kaplan-Meier estimate.
† Based on Cox proportional hazards model comparing the hazard functions associated with treatment groups, stratified by age (≤75 vs >75), diseases population (refractory to both Lenalidomide and Bortezomib vs not refractory to both drugs), and prior number of antimyeloma therapy (=2 vs >2), stratification factors for the trial.
‡ The p-value is based on a stratified log-rank test with the same stratification factors as the above Cox model.
§ 53% of patients in the High-dose Dex arm subsequently received POMALYST.
¶ Based on Cox proportional hazards model (unstratified) comparing the hazard functions associated with treatment groups.
# The p-value is based on an unstratified log-rank test.
Þ Alpha control for PFS and OS.
Figure 1. Progression Free Survival Based on IRAC Review of Response by IMWG Criteria (Stratified Log Rank Test) (ITT Population):
Data cut-off: 07 Sep 2012
Figure 2. Kaplan-Meier Curve of Overall Survival (ITT Population):
Data cutoff: 01 Mar 2013
14.2 Kaposi Sarcoma
The clinical trial 12-C-0047 (NCT01495598), was an open label, single center, single arm clinical study that evaluated the safety and efficacy of POMALYST in patients with Kaposi sarcoma (KS). A total of 28 patients (18 HIV-positive, 10 HIV-negative) received POMALYST 5 mg orally once daily on Days 1 through 21 of each 28-day cycle until disease progression or unacceptable toxicity. All HIV-positive patients continued highly active antiretroviral therapy (HAART). The trial excluded patients with symptomatic pulmonary or visceral KS, history of venous or arterial thromboembolism, or procoagulant disorders. Patients received thromboprophylaxis with aspirin 81 mg once daily throughout therapy.
The median age was 52.5 years, all were male, 75% were White, and 14% Black or African American. Seventy-five percent of patients had advanced disease (T1) at the time of enrollment, 11% had ≥50 lesions, and 75% had received prior chemotherapy.
The major efficacy outcome measure was overall response rate (ORR), which included complete response (CR), clinical complete response (cCR), and partial response (PR). Response was assessed by the investigator according to the AIDS Clinical Trial Group (ACTG) Oncology Committee response criteria for KS. The median time to first response was 1.8 months (0.9 to 7.6). Efficacy results are presented in Table 11.
Table 11. Trial 12-C-0047 Results:
| All Patients N=28 | HIV-Positive N=18 | HIV-Negative N=10 | |
|---|---|---|---|
| ORR*, n (%) | 20 (71) | 12 (67) | 8 (80) |
| [95% CI] | [51, 87] | (41, 87) | (44, 98) |
| CR*, n (%) | 4 (14) | 3 (17) | 1 (10) |
| PR, n (%) | 16 (57) | 9 (50) | 7 (70) |
| Duration of Response, KS†, | 12.1 | 12.5 | 10.5 |
| Median in months [95% CI]‡ | [7.6, 16.8] | [6.5, 24.9] | [3.9, 24.2] |
| Duration of Response, KS (%) | |||
| Percent greater than 12 months | 50 | 58 | 38 |
| Percent greater than 24 months | 20 | 17 | 25 |
CI: confidence interval, ORR: overall response rate, CR: complete response, PR: partial response
* CR includes one HIV-negative patient who achieved a cCR.
† Calculated as date of first documented response to date of first documented disease progression, receipt of new treatment or second course of treatment, or death due to any cause, whichever occurs first. Median estimate is from Kaplan-Meier analysis.
‡ From Kaplan-Meier analysis.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.