POMBILITI Powder for concentrate for solution for infusion Ref.[50960] Active ingredients: Cipaglucosidase alfa
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Amicus Therapeutics Europe Limited, Block 1, Blanchardstown Corporate Park, Ballycoolin Road, Blanchardstown, Dublin, D15 AKK1, Ireland e-mail: info@amicusrx.co.uk
4.1. Therapeutic indications
Pombiliti (cipaglucosidase alfa) is a long-term enzyme replacement therapy used in combination with the enzyme stabiliser miglustat for the treatment of adults with late-onset Pompe disease (acid α-glucosidase [GAA] deficiency).
4.2. Posology and method of administration
Treatment should be supervised by a physician experienced in the management of patients with Pompe disease or other inherited metabolic or neuromuscular diseases.
Cipaglucosidase alfa must be used in combination with miglustat 65 mg hard capsules. Because of this, the summary of product characteristics (SmPC) for miglustat 65 mg hard capsules should be consulted before taking cipaglucosidase alfa concerning number of capsules (based on body weight), dose time, and fasting.
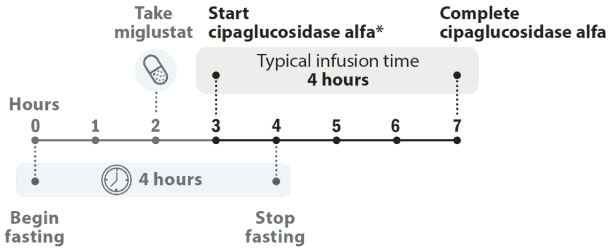
Posology
The recommended dose of cipaglucosidase alfa is 20 mg/kg of body weight every other week. The Pombiliti infusion should start 1 hour after taking miglustat capsules. In the event of infusion delay, the start of infusion should not exceed 3 hours from taking miglustat.
Figure 1. Dose timeline:
* The cipaglucosidase alfa infusion should start 1 hour after taking miglustat capsules. In the event of infusion delay, the start of infusion should not exceed 3 hours from taking miglustat.
Patient response to treatment should be routinely evaluated based on a comprehensive evaluation of all clinical manifestations of the disease. In case of an insufficient response or intolerable safety risks, discontinuation of cipaglucosidase alfa in combination with miglustat treatment should be considered, see section 4.4. Both medicinal products should either be continued or discontinued.
Switching patients from another enzyme replacement therapy (ERT)
If the patient is switching from another ERT to cipaglucosidase alfa in combination with miglustat therapy, the patient can be started with cipaglucosidase alfa-miglustat therapy at the next scheduled dosing time (i.e. approximately 2 weeks after the last ERT administration).
Patients who have switched from another ERT to cipaglucosidase alfa in combination with miglustat therapy should be advised to continue with any premedications used with the previous ERT therapy to minimise infusion-associated reactions (IARs). Depending on tolerability, premedication may be modified, see section 4.4.
Missed dose
If the cipaglucosidase alfa infusion cannot be started within 3 hours of oral administration of miglustat, reschedule treatment of cipaglucosidase alfa and miglustat at least 24 hours after taking miglustat. If cipaglucosidase alfa and miglustat are both missed, treatment should occur as soon as possible.
Special populations
Elderly
There is limited experience with the use of cipaglucosidase alfa in combination with miglustat therapy in patients above the age of 65 years old. There is no dose adjustment required in elderly patients, see section 5.2.
Renal and hepatic impairment
The safety and efficacy of cipaglucosidase alfa in combination with miglustat therapy have not been evaluated in patients with renal and/or hepatic impairment. When administering every other week, increased plasma miglustat exposure as a result of moderate or severe renal or hepatic impairment is not expected to appreciably impact cipaglucosidase alfa exposures and is not anticipated to affect efficacy and safety of cipaglucosidase alfa in a clinically meaningful manner. No dose adjustment is required in patients with renal impairment. The safety and efficacy of cipaglucosidase alfa in patients with hepatic impairment have not been evaluated and no specific dose regimen can be recommended for these patients.
Paediatric population
The safety and efficacy of cipaglucosidase alfa in combination with miglustat therapy in paediatric patients less than 18 years old have not yet been established. No data are available.
Method of administration
Cipaglucosidase alfa is to be administered by intravenous infusion.
Infusion of the 20 mg/kg dose is normally administered over the course of 4 hours if tolerated. Infusion should be administered in a stepwise manner. An initial cipaglucosidase alfa infusion rate of 1 mg/kg/hr is recommended. This infusion rate may be gradually increased by 2 mg/kg/hr approximately every 30 minutes if there are no signs of IARs until a maximum infusion rate of 7 mg/kg/hr is reached. The rate of infusion should be guided by the patient’s previous experience during infusion. The infusion rate may be slowed or temporarily stopped in the event of mild to moderate IARs. In the event of severe allergic, anaphylaxis, serious or severe IARs, the administration should immediately be discontinued, and appropriate medical treatment should be initiated, see sections 4.3 and 4.4.
Home infusion
Infusion of cipaglucosidase alfa at home may be considered for patients who are tolerating their infusions well and have no history of moderate or severe IARs for a few months. The decision to have a patient move to home infusion should be made after evaluation and upon recommendation by the treating physician. A patient’s underlying co-morbidities and ability to adhere to the home infusion requirements need to be taken into account when evaluating the patient for eligibility to receive home infusion. The following criteria should be considered:
- The patient must have no ongoing concurrent condition that, in the opinion of the physician, may affect patient’s ability to tolerate the infusion.
- The patient is considered medically stable. A comprehensive evaluation must be completed before the initiation of home infusion.
- The patient must have received cipaglucosidase alfa infusions supervised by a physician with expertise in management of Pompe patients for a few months that could be in a hospital or in another appropriate setting of outpatient care. Documentation of a pattern of well-tolerated infusions is a prerequisite for the initiation of home infusion.
- The patient must be willing and able to comply with home infusion procedures.
- Home infusion infrastructure, resources, and procedures, including training, must be established and available to the healthcare professional. The healthcare professional should be always available during the home infusion and for a specified time after infusion, depending on patient’s tolerance prior to starting home infusion.
If the patient experiences adverse reactions during the home infusion, the infusion process should be stopped immediately, and appropriate medical treatment should be initiated (see section 4.4). Subsequent infusions may need to occur in a hospital or in an appropriate setting of outpatient care until no such adverse reaction is present. Dose and infusion rate must not be changed without consulting the responsible physician.
The reconstituted product prior to dilution appears as a clear to opalescent colourless to slightly yellow solution. For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6.
4.9. Overdose
No doses of cipaglucosidase alfa in excess of 20 mg/kg body weight have been studied and no experience with accidental overdose have been observed to inform management of overdose. For management of adverse reactions, see sections 4.4 and 4.8.
6.3. Shelf life
Unopened container:
3 years.
Reconstituted medicinal product:
After reconstitution, chemical, physical, and microbiological in-use stability has been demonstrated for 24 hours at 2°C to 8°C.
From a microbiological point of view, the reconstituted product should be used immediately. If not used for dilution immediately, in-use storage times and conditions prior to dilution are the responsibility of the user and would normally not be longer than 24 hours at 2°C to 8°C.
Diluted medicinal product:
After dilution after reconstitution, chemical, physical, and microbiological in-use stability has been demonstrated between 0.5 mg/mL and 4 mg/mL for 24 hours at 2°C to 8°C, followed by 6 hours at room temperature (up to 25°C) to allow for infusion.
Use of aseptic techniques:
From a microbiological point of view, the medicinal product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 hours at 2°C to 8°C, followed by 6 hours at room temperature (up to 25°C) to allow for infusion.
Do not freeze the reconstituted vial or the diluted cipaglucosidase alfa solution in the bag for infusion.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C).
Keep the vial in the outer carton in order to protect from light.
For storage conditions after reconstitution and dilution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
105 mg of powder for concentrate for solution for infusion in a 20 mL neutral borosilicate clear Type I glass vial sealed with 20 mm chlorobutyl rubber stopper and with an aluminium over seal with dark grey plastic button.
Packs containing 1, 10, and 25 vials.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Preparation before the infusion:
Use aseptic technique.
Each vial of Pombiliti is for single-use only.
Calculating the dose:
Determine the number of Pombiliti vials to be reconstituted based on patient’s body weight.
1. Patient’s body weight (kg) x dose (mg/kg) = Patient dose (mg)
2. Patient’s dose (in mg) divided by 105 (mg per vial) = Number of vials to reconstitute
- If the number of vials includes a fraction, round up to the next whole number.
Example: in a 65 kg patient dosed at 20 mg/kg
- Patient dose (mg): 65 kg x 20 mg/kg = 1300 mg total dose
- Number of vials to reconstitute: 1300 divided by 105 mg per vial = 12.38 vials and round up to 13 vials.
- Remove 7.0 mL from each of the first 12 vials; 0.38 vial x 7.0 mL = 2.66 mL rounded to 2.7 mL from the 13th vial.
Items needed for reconstitution and dilution:
- Pombiliti 105 mg vials
- Sterile water for injections at room temperature of 20°C to 25°C
- Sodium chloride 9 mg/mL (0.9%) solution for injection at room temperature of 20°C to 25°C. Note: Choose a bag size based on the patient’s body weight.
- A needle of 18 gauge or lesser diameter
Activities before reconstitution:
- Pombiliti vials should be removed from the refrigerator (2° to 8°C) and allowed to come to room temperature (i.e. approximately 30 minutes at 20°C to 25°C).
- Do not use if the lyophilised powder is discoloured, or if the closure is damaged or the button of overseal is removed.
Reconstituting the lyophilised powder:
1. Reconstitute each vial by slowly adding 7.2 mL sterile water for injections dropwise down the inside of the vial rather than directly onto the lyophilised powder. Avoid forceful impact of sterile water for injections on the lyophilised powder and avoid foaming.
2. Tilt and roll each vial gently to dissolve the powder. Do not invert, swirl, or shake. Reconstitution of the lyophilised powder typically takes 2 minutes.
3. Perform an inspection of the reconstituted vials for particulate matter and discolouration. The reconstituted volume appears as a clear to opalescent, colourless to slightly yellow solution, free of foreign particles, and practically free of particles in the form of white to translucent particles. If upon immediate inspection foreign matter is observed or if the solution is discoloured, do not use.
4. Repeat above steps for the number of vials needed for dilution.
Dilution and preparation of the infusion bag:
1. Select an intravenous (IV) bag with sufficient volume to achieve a final target concentration range of 0.5 mg/mL to 4 mg/mL for the diluted cipaglucosidase alfa solution for IV infusion.
2. Remove airspace within the infusion bag. Remove an equal volume of sodium chloride 9 mg/mL (0.9%) solution for injections that will be replaced by the total volume (mL) of reconstituted cipaglucosidase alfa.
3. The reconstituted volume allows accurate withdrawal of 7.0 mL (equal to 105 mg) from each vial. Using a syringe with a needle diameter not larger than 18 gauge, slowly withdraw the reconstituted solution from the vials, including less than the 7.0 mL for the partial vial, until the patient’s dose is obtained. Avoid foaming in the syringe. Discard any remaining reconstituted solution in the last vial.
4. Slowly inject the reconstituted cipaglucosidase alfa solution directly into the sodium chloride 9 mg/mL (0.9%) solution for injection bag. Do not add directly into the air space that may remain within the infusion bag.
5. Gently invert or massage the bag to mix the diluted solution. Do not shake or excessively agitate the bag for infusion. Do not use a pneumatic tube to transport the infusion bag.
The infusion solution should be administered as close to after dilution preparation as possible at room temperature, see section 4.2.
Preparing for administration:
If it is not possible to start the infusion following dilution, the diluted solution is stable for up to 24 hours refrigerated at 2°C to 8°C. Storage at room temperature is not recommended, refer to the in-use stability storage conditions. Do not freeze or shake.
The sodium chloride 9 mg/mL (0.9%) solution for injections bag containing the diluted cipaglucosidase alfa is administered using an infusion pump.
Prior to infusion, inspect the infusion bag for foaming and if foaming is present, let foaming dissipate. Avoid shaking and handle infusion bag gently to prevent foaming.
An intravenous administration set should be used with an inline low protein binding 0.2-micron filter. If the IV-line blocks during infusion, change the filter.
Other medicinal products should not be infused in the same IV line as the diluted cipaglucosidase alfa solution.
Disposal:
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.