POTELIGEO Concentrate for solution for infusion Ref.[7600] Active ingredients: Mogamulizumab
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Kyowa Kirin Holdings B.V., Bloemlaan 2, 2132 NP, Hoofddorp, Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic and immunomodulating agents, monoclonal antibodies
ATC code: L01XC25
Mechanism of action
Mogamulizumab is a defucosylated, humanised IgG1 kappa immunoglobulin that selectively binds to CCR4, a G protein-coupled receptor for CC chemokines that is involved in the trafficking of lymphocytes to various organs including the skin, resulting in depletion of the target cells. CCR4 is expressed on the surface of some cancer cells including T cell malignancies, such as MF and SS in which CCR4 expression is inherent.
Clinical efficacy and safety
The efficacy of mogamulizumab in the treatment of patients with mycosis fungoides (MF) or Sézary syndrome (SS) was established in a Phase 3, multicentre, open-label, study (0761-010) of 372 adult patients randomised 1:1 to treatment with either mogamulizumab or vorinostat. Each arm enrolled 186 patients. Mogamulizumab infusion was administered at a dose of 1 mg/kg once weekly for the first 28-day cycle (on Days 1, 8, 15 and 22), and on days 1 and 15 of subsequent 28-day cycles. Vorinostat was administered at a starting dose of 400 mg orally, once daily beginning on day 1 for 28-day cycles. Vorinostat patients with disease progression or unacceptable toxicities were permitted to cross over to mogamulizumab therapy. Crossover patients received up to 46 months of mogamulizumab therapy, as of December 2016 data cut. Treatment with mogamulizumab continued until disease progression or unacceptable toxicity. The trial excluded patients with active autoimmune diseases, central nervous system metastasis, and medical conditions that required systemic corticosteroids or other immunosuppressive medicinal products, or an active infection requiring therapy, including HIV, or hepatitis B or C. Patients with ECOG performance status ≥2 were also excluded. At study baseline, 38% had stage IB-II disease, 10% stage III, 52% stage IV. This study included patients regardless of their baseline level of CCR4 expression in skin biopsy.
The primary efficacy endpoint was progression-free survival (PFS) based on investigator assessment using a global composite response criteria that took into account all potentially affected disease compartments (skin, blood, lymph nodes and viscera). Response in skin and blood was evaluated every 4 weeks. Response in lymph nodes and viscera was evaluated at 4 weeks, then every 8 weeks in the first year, and then every 16 weeks thereafter.
All patients had a histologically confirmed diagnosis of mycosis fungoides (MF), 56.5%, 53.2%, or Sézary Syndrome (SS), 43.5%, 46.8%, in the mogamulizumab and vorinostat groups, respectively, and had received at least one prior systemic therapy. The most common prior systemic therapies used by subjects in Europe were bexarotene (70%), interferon (59%), methotrexate (49%), extracorporeal photopheresis (ECP) (31%) and gemcitabine/gemcitabine regimens (28%).
The median duration of exposure with mogamulizumab was 5.6 months (range: <1 to 45.3 months). 56% of patients received mogamulizumab for at least 6 cycles, and 25% of patients received mogamulizumab for at least 12 cycles.
Patients were a median age of 64 years at the time of screening (range 25 to 101 years), 49.5% were 65 years or older, and 58.1% were male.
CCR4 expression was assessed retrospectively on pretreatment skin biopsies (formalin fixed paraffin embedded) using immunohistochemistry. In the mogamulizumab arm, baseline CCR4 expression levels were available in 75% of patients (N=140). CCR4 was detected on ≥1% of lymphocytes in 100% of patients, and 96% (134/140) had CCR4 detected on ≥10% of skin lymphocytes.
Of the patients randomised to vorinostat, 136 patients (73.1%) crossed over to mogamulizumab during the study. Reasons for crossover to mogamulizumab were disease progression (109 patients) and treatment intolerance (27 patients). The number of infusions of mogamulizumab administered to crossover patients ranged from 1 to 94 (up to 46 months of treatment) as of the December 2016 datacut.
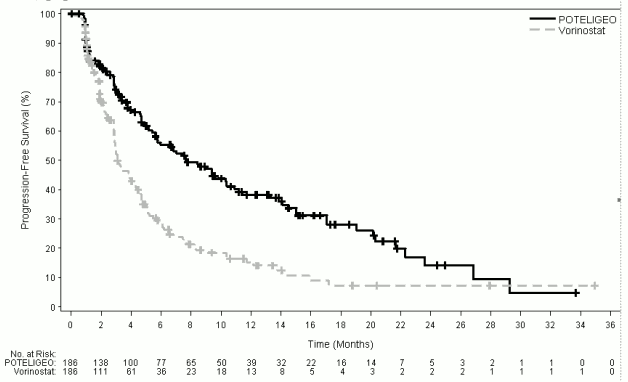
At 6, 12, 18 and 24 months after the start of randomised treatment, the percent of subjects alive without disease progression was higher for mogamulizumab (55.3%, 38.3%, 28.0%, and 14.1%, respectively) compared to vorinostat (28.8%, 15.3%, 7.2%, and 7.2%, respectively). Median PFS for the mogamulizumab group was 7.70 months (95% CI: 5.67, 10.33) and 3.10 months (95% CI: 2.87, 4.07) for the vorinostat group with resultant hazard ration of 0.53 (95% CI: 0.41, 0.69), p<0.0001 (2-sided, stratified log rank test).
The Kaplan-Meier curve for PFS is shown Figure 1.
Figure 1. Plot of Kaplan-Meier curve of progression-free survival by investigator's assessment (ITT) population:
Key secondary endpoints were overall response rate (ORR), ORR after crossover, duration of response (DOR), and changes from baseline of the Skindex-29 Symptoms and Functional Scales, and Functional Assessment of Cancer Therapy-General (FACT-G) Physical and Functional Well-being domains.
Overall response was reported as a composite score from measures in each compartment, and a response had to be demonstrated at two successive overall disease assessments (at least 8 weeks apart during the first year and 16 weeks apart thereafter) in order to be confirmed. Patients were included in the analysis for a specific compartment if they had presence of disease in that compartment at baseline, or had any post-baseline response assessment for that compartment.
Table 2 summarises ORR and DOR, and response by compartment. The study demonstrated statistically significant improvements in ORR and response by compartment in the blood, skin, and lymph nodes as compared to vorinostat. Response in the viscera could not be evaluated due to limited efficacy data in subjects with visceral involvement; the benefit-risk of mogamulizumab in subjects with visceral involvement is currently undetermined due to lack of data.
Table 2. Response during randomised treatment period in study 0761-010 (intent-to-treat):
| Mogamulizumab N=186 | Vorinostat N=186 | |
|---|---|---|
| Overall response rate (confirmed CR + PR, %) | 28.0 | 4.8 |
| 95% CI | (21.6, 35.0) | (2.2, 9.0) |
| P-valuea | <0.0001 | |
| Duration of response (months) | ||
| Median (95% CI) | 14.1 (9.4, 19.2) | 9.13 (4.7, - ) |
| Response by compartment | ||
| Blood | n=124 | n=125 |
| Response rate (confirmed CR + PR, %) | 66.9 | 18.4 |
| 95% CI | (57.9, 75.1) | (12.0, 26.3) |
| P-valuea | <0.0001 | |
| Skin | n=186 | n=186 |
| Overall response rate (confirmed CR + PR, %) | 41.9 | 15.6 |
| 95% CI | (34.8, 49.4) | (10.7, 21.6) |
| P-valuea | <0.0001 | |
| Lymph nodes | n=136 | n=133 |
| Overall response rate (confirmed CR + PR, %) | 15.4 | 3.8 |
| 95% CI | (9.8, 22.6) | (1.2, 8.6) |
| P-valuea | 0.0008 | |
| Viscera | n=6 | n=4 |
| Overall response rate (confirmed CR + PR, %) | 0 | 0 |
| 95% CI | (0.0, 45.9) | (0.0, 60.2) |
Note: Overall response rate is based on Global Composite Response score.
a P-value was obtained from Cochran-Mantel-Haenszel test adjusting for disease type, disease stage, and region.
CI=confidence interval; CR=complete response; PR=partial response
Treatment with mogamulizumab resulted in 8 confirmed complete responses (complete clearing of all affected compartments) compared with 0 patients on vorinostat: 4 of these 8 patients were initially randomized to mogamulizumab and 4 had crossed over to mogamulizumab during the study. Forty-one of the 136 cross-over patients (30.1%) responded with either partial or complete response with mogamulizumab.
There are limited efficacy data in patients with low (<10%) CCR4 expression in the skin. In Study 0761-010 there were 10/290 evaluable patients with CCR4 expression <10%, of which 6 were randomised to mogamulizumab, and 4 were randomised to vorinostat and subsequently crossed over to mogamulizumab. No confirmed responses were observed in these 10 subjects with low (<10%) CCR4 expression. Compartmental responses were seen in 3 of 10 evaluable subjects treated with mogamulizumab in the randomised or cross over phase.
Patients with stage IB/II disease treated with mogamulizumab had confirmed ORR of 17.6% compared to 8.3% for vorinostat, and compartment level (blood, skin, lymph node) response rates that were higher than those for vorinostat treated patients (Table 3). Overall, the median period of progression free survival for stage IB/II subjects treated with mogamulizumab was 4.7 months compared to 3.9 months for vorinostat-treated patients (Table 4). In patients with stage IB/II disease, given the limited number of subjects with a response and immaturity of the data, no conclusion on duration of response can be made.
Time to compartment level response in Stage IB/II patients was approximately 3 months, which is consistent with time to response for the ITT population overall (approximately 3 months). If a compartment level response or overall response is not observed after 3 months of treatment, discontinuation of treatment should be considered.
Table 3. Overall and Compartmental Response Rate in Early Disease Stages:
| Mogamulizumab | Vorinostat | Risk Diff (M vs. V) | |
|---|---|---|---|
| Disease stage IB/II | N=68 | N=72 | |
| Overall response rate (ORR), n (%) | 12 (17.6) | 6 (8.3) | 9.3 |
| Compartment: | |||
| Blood (n) | 17 | 23 | |
| Response Rate (n, %) | 8 (47.1) | 4 (17.4) | 29.7 |
| 95% CIa | (23.0, 72.2) | (5.0, 38.8) | (-2.2, 57.1) |
| Skin (n) | 68 | 72 | |
| Response Rate (n, %) | 19 (27.9) | 14 (19.4) | 8.5 |
| 95% CIa | (17.7, 40.1) | (11.1, 38.8) | (-8.3, 24.9) |
| Nodal (n) | 41 | 40 | |
| Response Rate (n, %) | 4 (9.8) | 1 (2.5) | 7.3 |
| 95% CIa | (2.7, 23.1) | (0.1, 13.2) | (-14.3, 28.6) |
M=mogamulizumab. V=vorinostat
Table 4. Progression Free Survival (PFS) by Treatment Group and Disease Stage (Randomised Treatment Period):
| Mogamulizumab | Vorinostat | P value | |
|---|---|---|---|
| PFS, months | |||
| ITT Population | 7.70 (5.67, 10.33) | 3.10 (2.87, 4.07) | <0.0001 |
| IB/II | 4.7 (2.9-7.47) | 3.9 (2.87-4.73) | 0.6790 |
| III/IV | 10.9 (7.03-15.03) | 3.0 (2.83-3.87) | <0.0001 |
ITT=intent to treat
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with mogamulizumab in all subsets of the paediatric population in cutaneous T-cell lymphoma (CTCL) (MF and SS are subtypes of CTCL). See section 4.2 for information on paediatric use.
Pharmacokinetic properties
The pharmacokinetics (PK) of mogamulizumab was evaluated in adult patients with T-cell leukaemia-lymphoma (ATL) and CTCL over a dose range of 0.01 to 1 mg/kg administered as multiple doses of mogamulizumab every week or every 2 weeks, and included the recommended 1.0 mg/kg dose and regimen (days 1, 8, 15 and 22 for the first 28-day cycle and on Days 1 and 15 for subsequent 28-day cycles). The population PK analysis included 444 patients receiving mogamulizumab in six clinical trials. The exposure to mogamulizumab increased proportionally with dose over the dose range of 0.1 to 1.0 mg/kg.
Absorption
Mogamulizumab is dosed via intravenous route and therefore is immediately and completely bioavailable.
Distribution
Based on a population PK analysis, the geometric mean [% coefficient of variation (CV%)] central volume of distribution (Vc) was 3.57 L (20.1%).
Biotransformation
The metabolic pathway of mogamulizumab has not been characterised. Mogamulizumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Elimination
Based on a population PK analysis, the geometric mean (% coefficient of variation [CV%]) clearance (CL) is 12.0 mL/h (83.7%) and geometric mean elimination half-life (t1/2) is 17 days (65.5%).
Linearity and accumulation
Mogamulizumab exhibits linear PK from the dose in a dose range of 0.01 mg/kg to 1 mg/kg. Based on a population PK analysis, the steady-state concentrations of mogamulizumab were reached after 12 weeks of repeated dosing when administered using the recommended regimen, and systemic accumulation was 1.7-fold. On a power model analysis, no deviation from dose proportionality was evident.
Renal impairment
The effect of renal impairment on the clearance of mogamulizumab was evaluated by a population PK analysis in patients with mild (creatinine clearance [CrCL] between 60 and 89; n=157), moderate (CrCL between 59 and 30; n=80), or severe renal impairment (CrCL less than 30 mL/min; n=2). No clinically important differences in the clearance of mogamulizumab were found between patients with mild to severe renal impairment and patients with normal renal function.
Hepatic impairment
The effect of hepatic impairment on the clearance of mogamulizumab was evaluated by a population PK analysis in patients with mild hepatic impairment (total bilirubin [TB] less than or equal to the upper limit of normal [ULN] and AST greater than ULN or TB less than 1 to 1.5 times ULN and any AST; n=80) or moderate (TB greater than 1.5 to 3 times ULN and any AST; n=3) hepatic impairment. No clinically important differences in the clearance of mogamulizumab were found between patients with mild to moderate hepatic impairment and patients with normal hepatic function. Mogamulizumab has not been studied in patients with severe hepatic impairment (TB greater than 3 times ULN and any AST).
Other special populations
The effects of various covariates on the PKs of mogamulizumab were assessed in population PK analyses. The following factors had no clinically important effect on the CL of mogamulizumab: age (range: 22 to 101 years), sex, ethnicity (other than Japanese, limited data are available in other ethnic populations), renal impairment, mild or moderate hepatic impairment, disease subtype (mycosis fungoides (MF) or Sézary Syndrome (SS)), degree of CCR4 expression or ECOG status, although it should be noted that patients with ECOG PS ≥2 were excluded from the clinical trials.
Pharmacokinetic/pharmacodynamic relationship(s)
Efficacy
Exposure-Response analysis indicated that efficacy was not correlated with mogamulizumab exposure in the pivotal study. Efficacy, as measured by improvement in PFS based on investigator assessment, was not associated with increasing mogamulizumab exposure.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeat dose toxicity. Carcinogenicity or genotoxicity studies have not been conducted with mogamulizumab. No specific studies have been conducted to evaluate potential effects on fertility.
No mogamulizumab-related toxic effects in the male and female reproductive organs were observed in repeat-dose toxicology studies in sexually mature monkeys up to 26 weeks.
In an animal reproductive and developmental toxicity study, administration of mogamulizumab to pregnant cynomolgus monkeys from the start of organogenesis through delivery did not show a potential for embryo-foetal lethality, teratogenicity, or foetal growth retardation. In general, IgG molecules are known to cross the placental barrier and mogamulizumab concentrations in foetus plasma were detected. Pharmacological activity of mogamulizumab was noted in foetuses as was evident from a decrease in CCR4 expressing lymphocytes.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.