PREVACID Delayed-release capsule / Delayed-release tablet Ref.[10609] Active ingredients: Lansoprazole
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
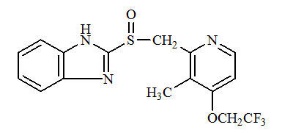
The active ingredient in PREVACID Delayed-Release Capsules and PREVACID SoluTab Delayed-Release Orally Disintegrating Tablets is lansoprazole, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F3N3O2S with a molecular weight of 369.37.
Lansoprazole has the following structure:
Lansoprazole is a white to brownish-white odorless crystalline powder which melts with decomposition at approximately 166°C. Lansoprazole is freely soluble in dimethylformamide; soluble in methanol; sparingly soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane and acetonitrile; very slightly soluble in ether; and practically insoluble in hexane and water.
Lansoprazole is stable when exposed to light for up to two months. The rate of degradation of the compound in aqueous solution increases with decreasing pH. The degradation half-life of the drug substance in aqueous solution at 25°C is approximately 0.5 hour at pH 5.0 and approximately 18 hours at pH 7.0.
PREVACID is supplied in delayed-release capsules and PREVACID SoluTab is supplied in delayed-release orally disintegrating tablets (SoluTab) for oral administration.
PREVACID is available in two dosage strengths: 15 and 30 mg of lansoprazole per capsule. Each delayed-release capsule contains enteric-coated granules consisting of 15 or 30 mg of lansoprazole (active ingredient) and the following inactive ingredients: sugar sphere, sucrose, methacrylic acid copolymer, low substituted hydroxypropyl cellulose, starch, magnesium carbonate, talc, polyethylene glycol, titanium dioxide, polysorbate 80, hydroxypropyl cellulose, colloidal silicon dioxide, D&C Red No. 28, FD&C Blue No. 1, FD&C Green No. 3?footnote?, and FD&C Red No. 40.
PREVACID SoluTab is available in two dosage strengths: 15 and 30 mg of lansoprazole per tablet. Each delayed-release orally disintegrating tablet contains enteric-coated microgranules consisting of 15 or 30 mg of lansoprazole (active ingredient) and the following inactive ingredients: mannitol, methacrylic acid, hydroxypropyl cellulose, lactose monohydrate-microcrystalline cellulose sphere, triethyl citrate, crospovidone, polyacrylate, magnesium carbonate, aspartame?footnote?, glyceryl monostearate, hypromellose, magnesium stearate, citric acid, titanium dioxide, talc, artificial strawberry flavor, polyethylene glycol, polysorbate 80 and ferric oxide.
| Dosage Forms and Strengths |
|---|
|
PREVACID delayed-release capsules:
PREVACID SoluTab delayed-release orally disintegrating tablets:
|
| How Supplied |
|---|
|
PREVACID SoluTab delayed-release orally disintegrating tablets, 15 mg, are white to yellowish white, round uncoated tablets containing orange to dark brown speckles, with "15" debossed on one side of the tablet. The 30 mg are white to yellowish white, round uncoated tablets containing orange to dark brown speckles, with "30" debossed on one side of the tablet. The tablets are available as follows: Overbagged with 10 tablets per bag, NDC 55154-0253-0 |
Drugs
| Drug | Countries | |
|---|---|---|
| PREVACID | Canada, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.