PREVYMIS Film-coated tablet Ref.[8782] Active ingredients: Letermovir
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antivirals for systemic use, direct acting antivirals
ATC code: J05AX18
Mechanism of action
Letermovir inhibits the CMV DNA terminase complex which is required for cleavage and packaging of viral progeny DNA. Letermovir affects the formation of proper unit length genomes and interferes with virion maturation.
Antiviral activity
The median EC50 value of letermovir against a collection of clinical CMV isolates in a cell-culture model of infection was 2.1 nM (range=0.7 nM to 6.1 nM, n=74).
Viral resistance
In cell culture
The CMV genes UL51, UL56, and UL89 encode subunits of CMV DNA terminase. CMV mutants with reduced susceptibility to letermovir have been confirmed in cell culture. EC50 values for recombinant CMV mutants expressing the substitutions map to pUL51 (P91S), pUL56 (C25F, S229F, V231A, V231L, V236A, T244K, T244R, L254F, L257F, L257I, F261C, F261L, F261S, Y321C, L328V, M329T, A365S, N368D), and pUL89 (N320H, D344E) were 1.6- to <10-fold higher than those for wild-type reference virus; these substitutions are not likely to be clinically relevant. EC50 values for recombinant CMV mutants expressing pUL51 substitution A95V or pUL56 substitutions N232Y, V236L, V236M, E237D, E237G, L241P, K258E, C325F, C325R, C325W, C325Y, R369G, R369M, R369S and R369T were 10- to 9 300-fold higher than those for the wild-type reference virus; some of these substitutions have been observed in patients who have experienced prophylaxis failure in clinical trials (see below).
In clinical studies
In a Phase 2b trial evaluating letermovir doses of 60, 120, or 240 mg/day or placebo for up to 84 days in 131 adult HSCT recipients, DNA sequence analysis of a select region of UL56 (amino acids 231 to 369) was performed on samples obtained from 12 letermovir-treated subjects who experienced prophylaxis failure and for whom samples were available for analysis. One subject (who received 60 mg/day) had a letermovir resistant genotypic variant (GV) (V236M).
In a Phase 3 trial (P001), DNA sequence analysis of the entire coding regions of UL56 and UL89 was performed on samples obtained from 40 letermovir-treated adult subjects in the FAS population who experienced prophylaxis failure and for whom samples were a vailable for analysis. Two subjects had letermovir resistant GVs detected, both with substitutions mapping to pUL56. One subject had the substitution V236M and the other subject had the substitution E237G. One additional subject, who had detectable CMV DNA at baseline (and was therefore not in the FAS population), had pUL56 substitutions, C325W and R369T, detected after discontinuing letermovir.
In a Phase 3 trial (P040), DNA sequence analysis of the entire coding regions of UL51, UL56 and UL89 was performed on samples obtained from 32 adult subjects (regardless of treatment group) who experienced prophylaxis failure or who discontinued early with CMV viremia. There were no letermovir resistance-associated substitutions detected above the validated assay limit of 5%.
In a Phase 3 trial (P002), DNA sequence analysis of the entire coding regions of UL51, UL56 and UL89 was performed on samples obtained from 52 letermovir-treated adult subjects who experienced CMV disease or who discontinued early with CMV viremia. There were no letermovir resistance-associated substitutions detected above the validated assay limit of 5%.
In a Phase 2b trial (P030), DNA sequence analysis of the entire coding regions of UL51, UL56 and UL89 was performed on samples obtained from 10 letermovir-treated paediatric subjects at a visit for evaluation of CMV infection. A total of 2 letermovir resistance-associated substitutions both mapping to pUL56 were detected in 2 subjects. One subject had the substitution R369S and the other subject had the substitution C325W.
Cross-resistance
Cross-resistance is not like ly with medicinal products with a different mechanism of action. Letermovir is fully active against viral populations with substitutions conferring resistance to CMV DNA polymerase inhibitors (ganciclovir, cidofovir, and foscarnet). A panel of recombinant CMV strains with substitutions conferring resistance to letermovir was fully susceptible to cidofovir, foscarnet and ganciclovir with the exception of a recombinant strain with the pUL56 E237G substitution which confers a 2.1-fold reduction in ganciclovir susceptibility relative to wild-type.
Cardiac electrophysiology
The effect of letermovir on doses up to 960 mg given intravenous ly on the QTc interval was evaluated in a randomi sed, single-dose, placebo- and active-controlled (moxifloxacin 400 mg oral) 4-period crossover thorough QT trial in 38 healthy adult subjects. Letermovir does not prolong QTc to any clinically relevant extent following the 960 mg intravenous dose with plasma concentrations approximately 2-fold higher than the 480 mg intravenous dose.
Clinical efficacy and safety
Adult CMV-seropositive recipients [R+] of an allogeneic hematopoietic stem cell transplant
P001: Prophylaxis through Week 14 (~100 days) post-HSCT
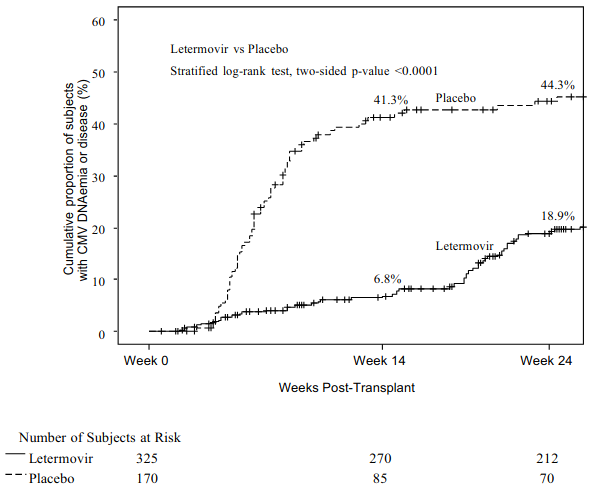
To evaluate letermovir prophylaxis as a preventive strategy for CMV infection or dise ase, the efficacy of letermovir was assessed in a multicentre, double-blind, placebo-controlled Phase 3 trial (P001) in adult CMV-seropositive recipients [R+] of an allogeneic HSCT. Subjects were randomi sed (2:1) to receive either letermovir at a dose of 480 mg once daily adjusted to 240 mg when co-administered with cyclosporine, or placebo. Randomisation was stratified by investigational site and risk (high vs. low) for CMV reactivation at the time of study entry. Letermovir was initiated after HSCT (Day 0-28 post-HSCT) and continued through Week 14 post-HSCT. Letermovir was administered either orally or intravenously; the dose of letermovir was the same regardless of the route of administration. Subjects were monitored through Week 24 post-HSCT for the primary efficacy endpoint with continued follow-up through Week 48 post-HSCT.
Subjects received CMV DNA monitoring weekly until post-HSCT week 14 and then every two weeks until post-HSCT week 24, with initiation of standard-of-care CMV pre-emptive therapy if CMV DNAemia was considered clinically significant. Subjects had c ontinued follow-up through Week 48 post-HSCT.
Among the 565 treated subjects, 373 subjects received letermovir (including 99 subjects who received at least one intravenous dose) and 192 received placebo (including 48 subjects who received at least one intravenous dose). The median time to starting letermovir was 9 days after transplantation. Thirty-seven percent (37%) of subjects were engrafted at baseline. The median age was 54 years (range: 18 to 78 years); 56 (15.0%) subjects were 65 years of age or older: 58% were male; 82% were White; 10% were Asian; 2% were Black or African; and 7% were Hispanic or Latino. At baseline, 50% of subjects received a myeloablative regimen, 52% were receiving cyclosporine, and 42% were receiving tacrolimus. The most common primary reasons for transplant were acute myelo id leukaemia (38%), myeloblastic syndrome (15%), and lymphoma (13%). Twelve percent (12%) of subjects were positive for CMV DNA at baseline.
At baseline, 31% of subjects were at high risk for reactivation as defined by one or more of the following criteria: Human Leucocyte Antigen (HLA)-related (sibling) donor with at least one mismatch at one of the following three HLA -gene loci: HLA-A, -B or –DR, haploidentical donor; unrelated donor with at least one mismatch at one of the following four HLA -gene loci: HLA-A, -B, -C and -DRB1; use of umbilical cord blood as stem cell source; use of ex vivo T-cell-depleted grafts; Grade 2 or greater Graft-Versus-Host Disease (GVHD), requiring systemic corticosteroids.
Primary efficacy endpoint:
The primary efficacy endpoint of clinically significant CMV infection in P001 was defined by the incidence of CMV DNAemia warranting anti-CMV pre-emptive therapy (PET) or the occurrence of CMV end-organ disease. The Non-Completer=Failure (NC=F) approach was used, where subjects who discontinued from the study prior to Week 24 post-HSCT or had a missing outcome at Week 24 post-HSCT were counted as failures.
Letermovir demonstrated superior efficacy over placebo in the analysis of the primary endpoint, as shown in Table 3. The estimated treatment difference of -23.5% was statistically significant (one-sided p-value <0.0001).
Table 3. P001: Efficacy results in HSCT recipients (NC=F Approach, FAS Population):
| Parameter | Letermovir (N=325) n (%) | Placebo (N=170) n (%) |
|---|---|---|
| Primary efficacy endpoint (Proportion of subjects who failed prophylaxis by Week 24) | 122 (37,5) | 103 (60,6) |
| Reasons for Failures† | ||
| Clinically significant CMV infection | 57 (17,5) | 71 (41,8) |
| CMV DNAemia warranting anti-CMV PET | 52 (16,0) | 68 (40,0) |
| CMV end-organ disease | 5 (1,5) | 3 (1,8) |
| Discontinued from study | 56 (17,2) | 27 (15,9) |
| Missing outcome | 9 (2,8) | 5 (2,9) |
| Stratum-adjusted treatment difference (Letermovir- Placebo)§ | ||
| Difference (95% CI) | -23,5 (-32,5, -14,6) | |
| p-value | <0,0001 |
† The categories of failure are mutually exclusive and based on the hierarchy of categories in the order listed.
§ 95% CIs and p-value for the treatment differences in percent response were calculated using stratum-adjusted Mantel-Haenszel method with the difference weighted by the harmonic mean of sample size per arm for each stratum (high or low risk). A 1-sided p-value ≤0.0249 was used for declaring statistical significance.
FAS=Full analysis set; FAS includes randomised subjects who received at least one dose of study medicine, and excludes subjects with detectable CMV DNA at baseline. Approach to handling missing values: Non-Completer=Failure (NC=F) approach. With NC=F approach, failure was defined as all subjects with clinically significant CMV infection or who prematurely discontinued from the study or had a missing outcome through Week 24 post-HSCT visit window.
N = number of subjects in each treatment group.
n (%) = Number (percent) of subjects in each sub-category.
Note: The proportion of subjects with detectable CMV viral DNA on Day 1 that developed clinically significant CMV infection in the letermovir group was 64.6% (31/48) compared to 90.9% (20/22) in the placebo group through Week 24 post-HSCT. The estimated difference (95% CI for the difference) was -26.1% (-45.9%, -6.3%), with a nominal one-sided p-value <0.0048.
Factors associated with CMV DNAemia after Week 14 post-HSCT among letermovir-treated subjects included high risk for CMV reactivation at baseline, GVHD, use of corticosteroids, and CMV negative donor serostatus.
Figure 1. P001: Kaplan-Meier plot of time to initiation of anti-CMV PET or onset of CMV end-organ disease through Week 24 post-transplant in HSCT recipients (FAS population):
There were no differences in the incidence of or time to engraftment between the letermovir and placebo groups.
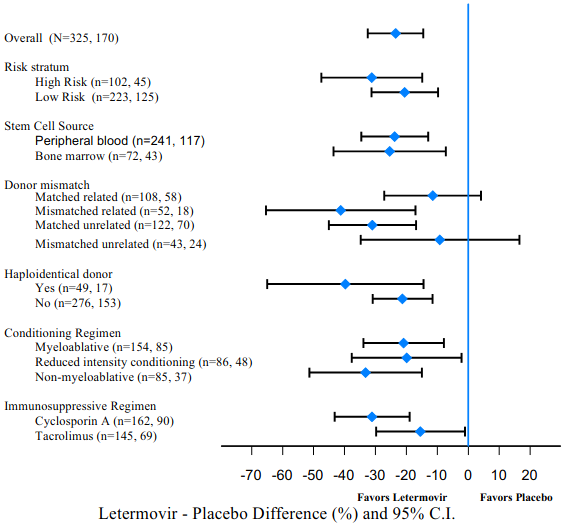
Efficacy consistently favoured letermovir across subgroups including low and high risk for CMV reactivation, conditioning regimens, and concomitant immunosuppressive regimens (see Figure 2).
Figure 2. P001: Forest plot of the proportion of subjects initiating anti-CMV PET or with CMV end-organ disease through Week 24 post-HSCT by selected subgroups (NC=F approach, FAS population):
NC=F, Non-Completer=Failure. With NC=F approach, subjects who discontinued from the study prior to Week 24 post-transplant or had a missing outcome at Week 24 post-transplant were counted as failures.
P040: Prophylaxis from Week 14 (~100 days) through Week 28 (~200 days) post-HSCT
The efficacy of extending letermovir prophylaxis from Week 14 (~100 days) through Week 28 (~200 days) post-HSCT in patients at risk for late CMV infection and disease was assessed in a multicentre, double-blind, placebo-controlled Phase 3 trial (P040) in adult CMV-seropositive recipients [R+] of an allogeneic HSCT. Eligible subjects who completed letermovir prophylaxis through ~100 days post-HSCT were randomised (2:1) to receive letermovir or placebo from Week 14 through Week 28 post-HSCT. Subjects were monitored through Week 28 post-HSCT for the primary efficacy endpoint with continued off-treatment follow-up through Week 48 post-HSCT.
Among the 218 treated subjects, 144 subjects received letermovir and 74 received placebo. The median age was 55 years (range: 20 to 74 years); 62% were male; 79% were white; 11% were Asian; 2% were Black; and 10% were Hispanic or Latino. The most common reasons for transplant were acute myeloid leukaemia (42%), acute lymphocytic leukaemia (15%), and myelodysplastic syndrome (11%).
At study entry, all subjects had risk factors for late CMV infection and disease, with 64% having two or more risk factors. The risk factors included: HLA-related (sibling) donor with at least one mismatch at one of the following three HLA-gene loci: HLA-A, -B or -DR; haploidentical donor; unrelated donor with at least one mismatch at one of the foll owing four HLA-gene loci: HLA-A, -B, -C and -DRB1; use of umbilical cord blood as stem cell source; use of ex vivo T-cell-depleted grafts; receipt of anti-thymocyte globulin; receipt of alemtuzumab; use of systemic prednisone (or equivalent) at a dose of ≥1 mg/kg of body weight per day.
Primary efficacy endpoint:
The primary efficacy endpoint of P040 was the incidence of clinically significant CMV infection through Week 28 post-HSCT. Clinically significant CMV infection was defined as the occurrence of either CMV end-organ disease, or initiation of anti-CMV PET based on documented CMV viremia and the clinical condition of the subject. The Observed Failure (OF) approach was used, where subjects who developed clinically significant CMV infection or discontinued prematurely from the study with viremia were counted as failures.
Letermovir demonstrated superior efficacy over placebo in the analysis of the primary endpoint, as shown in Table 4. The estimated treatment difference of -16.1% was statistically significant (one-sided p-value=0.0005). Efficacy consistently favored letermovir across subgroups based on subject characteristics (age, gender, race) and risk factors for late CMV infection and disease.
Table 4. P040: Efficacy results in HSCT recipients at risk for late CMV infection and disease (OF approach, FAS population):
| Parameter | Letermovir (~200 days letermovir) (N=144) n (%) | Placebo (~100 days letermovir) (N=74) n (%) |
|---|---|---|
| Failures* | 4 (2.8) | 14 (18.9) |
| Clinically significant CMV infection through Week 28† | 2 (1.4) | 13 (17.6) |
| Initiation of PET based on documented CMV viremia | 1 (0.7) | 11 (14.9) |
| CMV end-organ disease | 1 (0.7) | 2 (2.7) |
| Discontinued from study with CMV viremia before Week 28 | 2 (1.4) | 1 (1.4) |
| Stratum-adjusted treatment difference (letermovir (~200 days letermovir)-Placebo (~100 days letermovir))‡ | ||
| Difference (95% CI) | -16.1 (-25.8, -6.5) | |
| p-value | 0.0005 | |
* The categories of failure are mutually exclusive and based on the hierarchy of categories in the order listed.
† Clinically significant CMV infection was defined as CMV end-organ disease (proven or probable) or initiation of PET based on documented CMV viremia and the clinical condition of the subject.
‡ 95% CIs and p-value for the treatment differences in percent response were calculated using stratum-adjusted Mantel -Haenszel method with the difference weighted by the harmonic mean of sample size per arm for each stratum (haploidentical donor yes or no). A one -sided p-value ≤0.0249 was used for declaring statistical significance.
Approach to handling missing values: Observed Failure (OF) approach. With the OF approach, failure was defined as all subjects who developed clinically significant CMV infection or discontinued prematurely from the study with CMV viremia fro m Week 14 (~100 days) through Week 28 (~200 days) post-HSCT.
N=Number of subjects in each treatment group.
n (%)=Number (percent) of subjects in each sub-category.
P002: Adult CMV-seronegative recipients of a kidney transplant from a CMV-seropositive donor [D+/R-]
To evaluate letermovir prophylaxis as a preventive strategy for CMV disease in kidney transplant recipients, the efficacy of letermovir was assessed in a multicentre, double-blind, active comparator-controlled non-inferiority Phase 3 trial (P00 2) in adult kidney transplant recipients at high risk [D+/R-]. Subjects were randomi sed (1:1) to receive either letermovir or valganciclovir. Letermovir was given concomitantly with acyclovir. Valganciclovir was given concomitantly with a placebo to acyclovir. Randomisation was stratified by the use or non-use of highly cytolytic, anti-lymphocyte immunotherapy during induction. Letermovir or valganciclovir were initiated between Day 0 and Day 7 post-kidney transplant and continued through Week 28 (~200 days) post-transplant. Subjects were monitored through Week 52 post-transplant.
Among the 589 treated subjects, 292 subjects received letermovir and 297 received valganciclovir. The median age was 51 years (range: 18 to 82 years); 72% were male; 84% were White; 2% were Asian; 9% were Black; 17% were Hispanic or Latino; and 60% received a kidney from a deceased donor. The most common primary reasons for transplant were congenital cystic kidney disease (17%), hypertension (16%), and diabetes/diabetic nephropathy (14%).
Primary efficacy endpoint:
The primary efficacy endpoint of P002 was the incidence of CMV disease (CMV end-organ disease or CMV syndrome, confirmed by an independent adjudication committee) through Week 52 post-transplant. The OF approach was used, where subjects who discontinued prematurely from the study for any reason or were missing data at the timepoint were not considered failures.
Letermovir demonstrated non-inferiority to valganciclovir in the analysis of the primary endpoint, as shown in Table 5.
Table 5. P002 Efficacy results in kidney transplant recipients (OF approach, FAS population):
| Parameter | Letermovir (N=289) n (%) | Valganciclovir (N=297) n (%) |
|---|---|---|
| CMV disease* through Week 52 | 30 (10.4) | 35 (11.8) |
| Stratum-adjusted treatment difference (Letermovir-Valganciclovir)† Difference (95% CI) | -1.4 (-6.5, 3.8)‡ | |
* CMV disease cases confirmed by an independent adjudication committee.
† The 95% CIs for the treatment differences in percent response were calculated using stratum-adjusted Mantel-Haenszel method with the difference weighted by the harmonic mean of sample size per arm for each stratum (use/nonuse of highly cytolytic, anti-lymphocyte immunotherapy during induction).
‡ Based on a non-inferiority margin of 10%, letermovir is non-inferior to valganciclovir.
Approach to handling missing values: Observed F ailure (OF) approach. With OF approach, participants who discontinue prematurely from the study for any reason are not considered failures.
Note: Subjects randomised to the letermovir group were given acyclovir for herpes simplex virus (HSV) and varicella zoster virus (VZV) prophylaxis. Subjects randomi sed to the valganciclovir group were given a placebo to acyclovir.
N=number of subjects in each treatment group.
n (%)=Number (percent) of subjects in each sub-category.
Efficacy was comparable across all subgroups, including sex, age, race, region, and the use/non-use of highly cytolytic, anti -lymphocyte immunotherapy during induction.
Paediatric population
P030: Paediatric recipients of an allogeneic hematopoietic stem cell transplant
To evaluate letermovir prophylaxis as a preventive strategy for CMV infection or disease in paediatric transplant recipients, the efficacy of letermovir was assessed in a multicentre, open-label, single-arm Phase 2b trial (P030) in paediatric recipients of an allogeneic HSCT. Study drug was initiated after HSCT (Day 0-28 post-HSCT) and continued through Week 14 post-HSCT. Study drug was administered either orally or intravenously; the dose of letermovir was based on age, body weight and formulation.
Among the 63 treated subjects, 8 were 0 to less than 2 years of age, 27 were 2 to less than 12 years of age and 28 were 12 to less than 18 years of age. At baseline, 87% of subjects received a myeloablative regimen, 67% were receiving cyclosporine, and 27% were receiving tacrolimus. The most common primary reasons for transplant were acute myeloid leukaemia (18%) a nd aplastic anaemia (10%) in the overall population, and combined immunodeficiency (37.5%) and familial haemophagocytic lymphohistiocytosis (25.0%) in children less than 2 years of age.
Secondary efficacy endpoint
The efficacy endpoints of P030 were secondary and included the incidence of clinically significant CMV infection through Week 14 post-HSCT and through Week 24 post-HSCT. Clinically significant CMV infection was defined as the occurrence of either CMV end-organ disease, or initiation of anti-CMV PET based on documented CMV viremia and the clinical condition of the subject. The incidence of clinically significant CMV infection was 7.1% and 10.7% through Week 14 post-HSCT and Week 24 post-HSCT, respectively.
Pharmacokinetic properties
In healthy adult subjects, the pharmacokinetics of letermovir have been characterised following oral and intravenous administration. Letermovir exposure increased in a greater than dose-proportional manner with both oral or intravenous administration. The mechanism is likely saturation/autoinhibition of OATP1B1/3. The pharmacokinetics of letermovir have also been characterised following oral and intravenous administration in adult HSCT recipients (see Table 6) and paediatric HSCT recipients (see Table 8 and Table 9) and following oral administration in adult kidney transplant recipients (see Table 7).
Healthy adult subjects
The geometric mean steady-state AUC and Cmax values were 71 500 ng•hr/mL and 13 000 ng/mL, respectively, with 480 mg once daily oral letermovir.
Letermovir reached steady-state in 9 to 10 days with an accumulation ratio of 1.2 for AUC and 1 for Cmax.
Adult HSCT recipients
Letermovir AUC was estimated using population pharmacokinetic analyses using P001 Phase 3 data (see Table 6). Differences in exposure across treatment regimens are not clinically relevant; efficacy was consistent across the range of exposures observed in P001.
Table 6. Letermovir AUC (ng•hr/mL) values in HSCT Recipients:
| Treatment Regimen | Median (90% Prediction Interval)* |
|---|---|
| 480 mg Oral, no cyclosporine | 34 400 (16 900, 73 700) |
| 480 mg intravenous, no cyclosporine | 100 000 (65 300, 148 000) |
| 240 mg Oral, with cyclosporine | 60 800 (28 700, 122 000) |
| 240 mg intravenous, with cyclosporine | 70 300 (46 200, 106 000) |
* Population post-hoc predictions from the population PK analysis using Phase 3 data
Adult kidney transplant recipients
Letermovir AUC was estimated using population pharmacokinetic analysis using P002 Phase 3 data (see Table 7). Efficacy was consistent across the range of exposures observed in P002.
Table 7. Letermovir AUC (ng•hr/mL) values in kidney transplant recipients:
| Treatment Regimen | Median (90% Prediction Interval)* |
|---|---|
| 480 mg Oral, no cyclosporine | 62 200 (28 900, 145 000) |
| 240 mg Oral, with cyclosporine | 57 700 (26 900, 135 000) |
* Medians and 90% prediction intervals are based on simulations using the Phase 3 population PK model with inter-individual variability.
Note: PK of letermovir was not studied following intravenous administration in kidney transplant recipients; however, the projected AUC following intravenous administration is similar to the model predicted AUC following intravenous administration in HSCT recipients (see Table 6).
Absorption
In healthy adult subjects, letermovir was absorbed rapidly with a median time to maximum plasma concentration (Tmax) of 1.5 to 3.0 hours and declined in a biphasic manner. In adult HSCT recipients, bioavailability of letermovir was estimated to be approximately 35% with 480 mg once daily oral letermovir administered without cyclosporine. The inter-individual variability for bioavailability was estimated to be approximately 37%. In adult kidney transplant recipients, bioavailability of letermovir was estimated to be approximately 60% with 480 mg once daily oral letermovir administered without cyclosporine.
Effect of cyclosporine
In adult HSCT recipients, co-administration of cyclosporine increased plasma concentrations of letermovir due to inhibition of OATP1B. Bioavailability of letermovir was estimated to be approximately 85% with 240 mg once daily oral letermovir co-administered with cyclosporine in patients.
If letermovir is co-administered with cyclosporine, the recommended dose of letermovir is 240 mg once daily in adult and paediatric patients weighing at least 30 kg (see section 4.2). If oral letermovir is co-administered with cyclosporine in paediatric patients weighing less than 30 kg, the dose should be decreased (see section 4.2).
Effect of food
In healthy adult subjects, oral administration of 480 mg single dose of letermovir tablet with a standard high fat and high calorie meal did not have any effect on the overall exposure (AUC) and resulted in approximately 30% increase in peak levels (Cmax) of letermovir. Letermovir tablets may be administered orally with or without food as has been done in the clinical trials (see section 4.2).
In healthy adult subjects, oral administration of 240 mg single dose of letermovir granules with soft foods (pudding or applesauce) resulted in an approximately 13% and 20% increase in overall exposure (AUC) and resulted in approximately 25% and 33% increase in peak levels (Cmax) of letermovir. Letermovir granules may be administered with soft foods, as has been done in the paediatric trial (see section 4.2).
Distribution
Based on population pharmacokinetic analyses, the mean steady-state volume of distribution is estimated to be 45.5 L following intravenous administration in adult HSCT recipients.
Letermovir is extensively bound (98.2%) to human plasma proteins, independent of the concentration range (3 to 100 mg/L) evaluated, in vitro. Some saturation was observed at lower concentrations. Blood to plasma partitioning of letermovir is 0.56 and independent of the concentration range (0.1 to 10 mg/L) evaluated in vitro.
In preclinical distribution studies, letermovir is distributed to organs and tissues with the highest concentrations observed in the gastrointestinal tract, bile duct and liver and low concentrations in the brain.
Biotransformation
The majority of letermovir-related components in plasma is unchanged parent (96.6%). No major metabolites are detected in plasma. Letermovir is partly eliminated by glucuronidation mediated by UGT1A1/1A3.
Elimination
The mean apparent terminal half-life for letermovir is approximately 12 hours with 480 mg intravenous letermovir in healthy adult subjects. The major elimination pathways of letermovir is biliary excretion as well as direct glucuronidation. The process involves the hepatic uptake transporters OATP1B1 and 3 followed by UGT1A1/3 catalysed glucuronidation.
Based on population pharmacokinetic analyses, letermovir steady-state apparent CL is estimated to be 4.84 L/hr following intravenous administration of 480 mg in adult HSCT recipients. The inter-individual variability for CL is estimated to be 24.6%.
Excretion
After oral administration of radio-labeled letermovir, 93.3% of radioactivity was recovered in faeces. The majority of letermovir was biliary excreted as unchanged pa rent with a minor amount (6% of dose) as an acyl-glucuronide metabolite in faeces. The acyl-glucuronide is unstable in faeces. Urinary excretion of letermovir was negligible (<2% of dose).
Pharmacokinetics in special populations
Hepatic impairment
Letermovir unbound AUC was approximately 81% - and 4-fold higher in adult subjects with moderate (Child-Pugh Class B [CP-B], score of 7-9) and severe (Child-Pugh Class C [CP-C], score of 10-15) hepatic impairment, respectively, compared to healthy adult subjects. The changes in letermovir exposure in adult subjects with moderate hepatic impairment are not clinically relevant. Marked increases in letermovir unbound exposure are anticipated in patients with moderate hepatic impairment combined with moderate or severe renal impairment (see section 4.2).
Renal impairment
Clinical study in a renally impaired population:
Letermovir unbound AUC was approximately 115 – and 81% higher in adult subjects with moderate (eGFR of 31.0 to 56.8 mL/min/1.73m²) and severe (eGFR of 11.9 to 28.1 mL/min/1.73m²) renal impairment, respectively, compared to healthy adult subjects. The changes in letermovir exposure due to moderate or severe renal impairment are not considered to be clinically relevant. Subjects with ESRD have not been studied.
Post-kidney transplant (P002):
Based on population pharmacokinetic analysis, letermovir AUC was approximately 12%, 27% and 35% higher in adult subjects with mild ( CrCl greater than or equal to 60 to less than 90 mL/min), moderate (CrCl greater than or equal to 30 to less than 60 mL/min) and severe (CrCl greater than or equal to 15 to less than 30 mL/min) renal impairment, respectively, compared to adult subjects with CrCl greater than or equal to 90 mL/min. These changes are not considered to be clinically relevant.
Weight
Based on population pharmacokinetic analyses in healthy adult subjects, letermovir AUC is estimated to be 18.7% lower in subjects weighing 80-100 kg compared to subjects weighing 67 kg. Based on population pharmacokinetic analys is in adult kidney transplant recipients (P002), letermovir AUC is estimated to be 26% lower in subjects weighing greater than 80 kg compared to subjects weighing less than or equal to 80 kg. These differences are not clinically relevant.
Race
Based on population pharmacokinetic analyses in healthy adult subjects, letermovir AUC is estimated to be 33.2% higher in Asians compared to Whites. This change is not clinically relevant.
Gender
Based on population pharmacokinetic analyses, there is no difference in letermovir pharmacokinetics in adult females compared to males.
Elderly
Based on population pharmacokinetic analyses, there is no effect of age on letermovir pharmacokinetics. No dose adjustment is required based on age.
Paediatric population
Letermovir AUC in paediatric HSCT recipients was estimated via population pharmacokinetic analysis using observed PK data from study P030 (see Table 8 and Table 9). Exposures for paediatric HSCT recipients across body weight bands are within the range of exposures achieved in the adult HSCT reference exposures (see Table 6).
Table 8. Letermovir AUC (ng•hr/mL) values following oral administration in paediatric HSCT recipients:
| Body weight | Oral dose, no cyclosporine | Median (90% prediction interval)* | Oral dose, with cyclosporine | Median (90% prediction interval)* |
|---|---|---|---|---|
| 30 kg and above | 480 mg | 39 100 (18 700-81 300) | 240 mg | 49 100 (23 200-104 000) |
| 15 kg to less than 30 kg | 240 mg | 38 900 (20 200-74 300) | 120 mg | 51 000 (26 600-98 200) |
| 7.5 kg to less than 15 kg | 120 mg | 32 000 (16 700-59 300) | 60 mg | 41 600 (22 300-81 100) |
| 5 kg to less than 7.5 kg | 80 mg | 30 600 (16 200-55 000) | 40 mg | 39 000 (20 600-72 000) |
* Medians and 90% prediction intervals are based on simulations using the paediatric HSCT population PK model with inter-individual variability.
Table 9. Letermovir AUC (ng•hr/mL) values following intravenous administration in paediatric HSCT recipients:
| Body weight | Intravenous dose, no cyclosporine | Median (90% prediction interval)* | Intravenous dose, with cyclosporine | Median (90% prediction interval)* |
|---|---|---|---|---|
| 30 kg and above | 480 mg | 111 000 (55 700-218 000) | 240 mg | 59 800 (28 400-120 000) |
| 15 kg to less than 30 kg | 120 mg | 57 200 (29 700-113 000) | 120 mg | 61 100 (29 900-121 000) |
| 7.5 kg to less than 15 kg | 60 mg | 46 000 (24 300-83 900) | 60 mg | 49 200 (25 800-93 800) |
| 5 kg to less than 7.5 kg | 40 mg | 43 400 (24 300-81 000) | 40 mg | 45 900 (24 900-82 200) |
* Medians and 90% prediction intervals are based on simulations using the paediatric HSCT population PK model with inter-individual variability.
Preclinical safety data
General toxicity
Irreversible testicular toxicity was noted only in rats at systemic exposures (AUC) ≥3-fold the exposures in humans at the recommended human dose (RHD). This toxicity was characterised by seminiferous tubular degeneration, and oligospermia and cell debris in the epididymides, with decreased testic ular and epididymides weights. There was no testicular toxicity in rats at exposures (AUC) similar to the exposures in humans at the RHD. Testicular toxicity was not observed in mice and monkeys at the highest doses tested at exposures up to 4-fold and 2-fold, respectively, the exposures in humans at the RHD. The relevance to humans is unknown.
Carcinogenesis
A 6-month oral carcinogenicity study in RasH2 transgenic (Tg.RasH2) mice showed no evidence of human-relevant tumorigenesis up to the highest doses tested, 150 mg/kg/day and 300 mg/kg/day in males and females, respectively.
Mutagenesis
Letermovir was not genotoxic in a battery of in vitro or in vivo assays, including microbial mutagenesis assays, chromosomal aberration in Chinese Hamster Ovary cells, and in an in vivo mouse micronucleus study.
Reproduction
Fertility
In the fertility and early embryonic development studies in the rat, there were no effects of letermovir on female fertility. In male rats, reduced sperm concentration, reduced sperm motility, and decreased fertility were observed at systemic exposures ≥3-fold the AUC in humans at the RHD (see General toxicity).
In monkeys administered letermovir, there was no evidence of testicular toxicity based on histopathologic evaluation, measurement of testicular size, blood hormone analysis (follicle stimulating hormone, inhibin B and testosterone) and sperm evaluation (sperm count, motility and morphology) at systemic exposures approximately 2-fold the AUC in humans at the RHD.
Development
In rats, maternal toxicity (including decrease in body weight gain) was noted at 250 mg/kg/day (approximately 11 -fold the AUC at the RHD); in the offspring, decreased foetal weight with delayed ossification, slightly oedematous foetuses, and increased incidence of shortened umbilical cords and of variations and malformations in the vertebrae, ribs, and pelvis were observed. No maternal or developmental effects were noted at the dose of 50 mg/kg/day (approximately 2.5-fold the AUC at the RHD).
In rabbits, maternal toxicity (including mortality and abortions) was noted at 225 mg/kg/day (approximately 2-fold the AUC at the RHD); in the offspring, an increased in cidence of malformations and variations in the vertebrae and ribs were observed.
In the pre- and post-natal developmental study, letermovir was administered orally to pregnant rats. There was no developmental toxicity observed up to the highest exposure tested (2-fold the AUC at the RHD).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.