PRILOSEC Granule for delayed-release oral suspension Ref.[10610] Active ingredients: Omeprazole
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
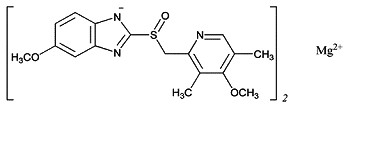
The active ingredient in PRILOSEC (omeprazole magnesium) for delayed-release oral suspension, is 5-Methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, magnesium salt (2:1).
Omeprazole magnesium is a white to off white powder with a melting point with degradation at 200°C. The salt is slightly soluble (0.25 mg/mL) in water at 25°C, and it is soluble in methanol. The half-life is highly pH dependent.
The empirical formula for omeprazole magnesium is (C17H18N3O3S)2 Mg, the molecular weight is 713.12 and the structural formula is:
Each packet of PRILOSEC for delayed-release oral suspension contains either 2.5 mg or 10 mg of omeprazole (equivalent to 2.8 mg or 11.2 mg of omeprazole magnesium trihydrate), in the form of enteric-coated granules with the following inactive ingredients: glyceryl monostearate, hydroxypropyl cellulose, hypromellose, magnesium stearate, methacrylic acid copolymer C, polysorbate, sugar spheres, talc, and triethyl citrate, and also inactive granules. The inactive granules are composed of the following ingredients: citric acid, crospovidone, dextrose, hydroxypropyl cellulose, iron oxide and xanthan gum. The omeprazole granules and inactive granules are constituted with water to form a suspension and are given by oral, nasogastric, or direct gastric administration.
| Dosage Forms and Strengths |
|---|
|
PRILOSEC For Delayed-Release Oral Suspension: 2.5 mg and 10 mg omeprazole in unit dose packets containing a fine yellow powder, consisting of white to brownish omeprazole magnesium granules and pale yellow inactive granules. |
| How Supplied |
|---|
|
PRILOSEC (omeprazole magnesium) for delayed-release oral suspension, 2.5 mg or 10 mg omeprazole, is supplied as a unit dose packet containing a fine yellow powder, consisting of white to brownish omeprazole magnesium granules and pale yellow inactive granules. PRILOSEC unit dose packets are supplied as follows: NDC 70515-625–01 unit dose packages of 30: 2.5 mg packets NDC 70515-610–01 unit dose packages of 30: 10 mg packets Manufactured for: Covis Pharma, Zug, 6300 Switzerland |
Drugs
| Drug | Countries | |
|---|---|---|
| PRILOSEC | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.