PRISTIQ Extended-release tablet Ref.[10909] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
The exact mechanism of the antidepressant action of desvenlafaxine is unknown, but is thought to be related to the potentiation of serotonin and norepinephrine in the central nervous system, through inhibition of their reuptake. Non-clinical studies have shown that desvenlafaxine is a potent and selective serotonin and norepinephrine reuptake inhibitor (SNRI).
12.2. Pharmacodynamics
Desvenlafaxine lacked significant affinity for numerous receptors, including muscarinic-cholinergic, H1-histaminergic, or α 1-adrenergic receptors in vitro. Desvenlafaxine also lacked monoamine oxidase (MAO) inhibitory activity.
ECG changes
Electrocardiograms were obtained from 1,492 desvenlafaxine treated patients with major depressive disorder and 984 placebo-treated patients in clinical studies lasting up to 8 weeks. No clinically relevant differences were observed between desvenlafaxine treated and placebo-treated patients for QT, QTc, PR, and QRS intervals. In a thorough QTc study with prospectively determined criteria, desvenlafaxine did not cause QT prolongation. No difference was observed between placebo and desvenlafaxine treatments for the QRS interval.
12.3. Pharmacokinetics
The single-dose pharmacokinetics of desvenlafaxine are linear and dose-proportional in a dose range of 50 to 600 mg (1 to 12 times the recommended approved dosage) per day. With once-daily dosing, steady-state plasma concentrations are achieved within approximately 4 to 5 days. At steady-state, multiple-dose accumulation of desvenlafaxine is linear and predictable from the single-dose pharmacokinetic profile.
Absorption
The absolute oral bioavailability of PRISTIQ after oral administration is about 80%.
Effect of Food
Ingestion of a high-fat meal (800 to 1000 calories) increased desvenlafaxine Cmax about 16% and had no effect on AUC.
Distribution
Steady-state volume of distribution of desvenlafaxine is 3.4 L/kg. Plasma protein binding of desvenlafaxine is 30% and is independent of drug concentration.
Elimination
Metabolism
Desvenlafaxine is primarily metabolized by conjugation (mediated by UGT isoforms) and, to a minor extent, through oxidative metabolism. CYP3A4 mediates the oxidative metabolism (N-demethylation) of desvenlafaxine. The CYP2D6 metabolic pathway is not involved. The pharmacokinetics of desvenlafaxine was similar in subjects with CYP2D6 poor and extensive metabolizer phenotype.
Excretion
Approximately 45% of desvenlafaxine is excreted unchanged in urine at 72 hours after oral administration. Approximately 19% of the administered dose is excreted as the glucuronide metabolite and <5% as the oxidative metabolite (N,O-didesmethylvenlafaxine) in urine.
Specific Populations
No clinically significant differences in the exposures of desvenlafaxine were observed based on ethnicity (White, Black, Hispanic).
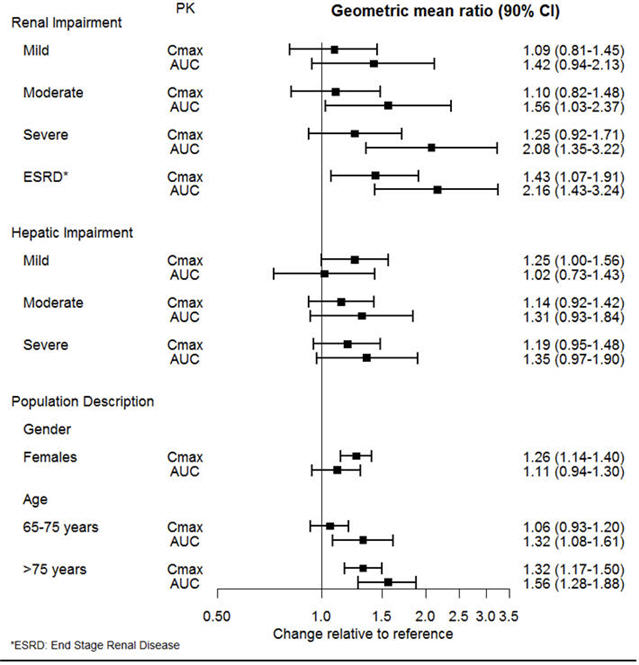
The effect of intrinsic patient factors on the pharmacokinetics of desvenlafaxine is presented in Figure 1.
Figure 1. Impact of Intrinsic Factors (Renal, Hepatic Impairment and Population Description) on Desvenlafaxine Pharmacokinetics:
Drug Interaction Studies
Clinical Studies
Other Drugs on PRISTIQ:
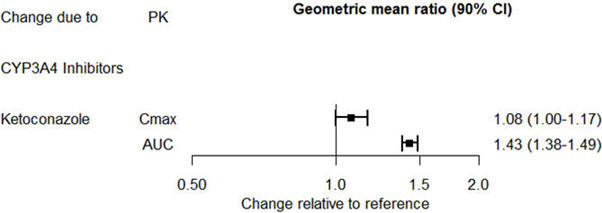
The effect of ketoconazole on the exposures of desvenlafaxine is summarized in Figure 2.
Figure 2. Effect of Other Drugs on Desvenlafaxine Pharmacokinetics:
PRISTIQ on Other Drugs:
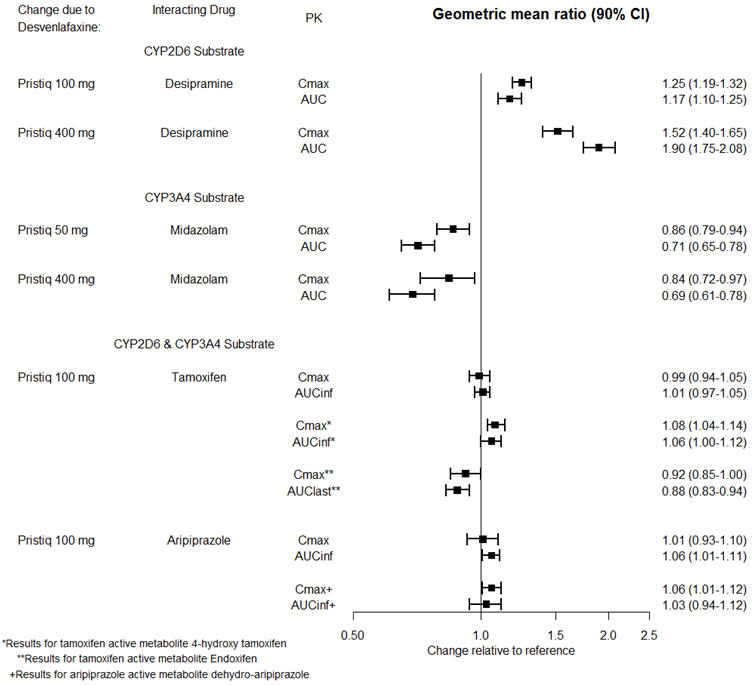
The effects of PRISTIQ on the exposures of other drugs are summarized in Figure 3.
Figure 3. Effects of PRISTIQ on Pharmacokinetics of Other Drugs:
In Vitro Studies
Based on in vitro data, drugs that inhibit CYP isozymes 1A1, 1A2, 2A6, 2D6, 2C8, 2C9, 2C19, and 2E1 are not expected to have significant impact on the pharmacokinetic profile of desvenlafaxine.
Desvenlafaxine does not inhibit CYP1A2, 2A6, 2C8, 2C9, 2C19 CYP2D6, or CYP3A4 isozymes. Desvenlafaxine does not induce CYP3A4 either.
Desvenlafaxine is not a substrate or an inhibitor for the P-glycoprotein (P-gp) transporter.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Desvenlafaxine succinate administered by oral gavage to mice and rats for 2 years did not increase the incidence of tumors in either study.
Mice received desvenlafaxine succinate at dosages up to 500/300 mg/kg/day (dosage lowered after 45 weeks of dosing). The AUC exposure at 300 mg/kg/day dose is estimated at 10 times the AUC exposure at an adult human dose of 100 mg per day.
Rats received desvenlafaxine succinate at dosages up to 300 mg/kg/day (males) or 500 mg/kg/day (females). The AUC exposure at the highest dose is estimated at 11 (males) or 26 (females) times the AUC exposure at an adult human dose of 100 mg per day.
Mutagenesis
Desvenlafaxine was not mutagenic in the in vitro bacterial mutation assay (Ames test) and was not clastogenic in an in vitro chromosome aberration assay in cultured CHO cells, an in vivo mouse micronucleus assay, or an in vivo chromosome aberration assay in rats. Additionally, desvenlafaxine was not genotoxic in the in vitro CHO mammalian cell forward mutation assay and was negative in the in vitro BALB/c-3T3 mouse embryo cell transformation assay.
Impairment of Fertility
When desvenlafaxine succinate was administered orally to male and female rats, fertility was reduced at the high dose of 300 mg/kg/day, which is 10 (males) and 19 (females) times the AUC exposure at an adult human dose of 100 mg per day. There was no effect on fertility at 100 mg/kg/day, which is 3 (males) or 5 (females) times the AUC exposure at an adult human dose of 100 mg per day. These studies did not address reversibility of the effect on fertility. The relevance of these findings to humans is not known.
14. Clinical Studies
Major Depressive Disorder
The efficacy of PRISTIQ as a treatment for depression was established in four 8-week, randomized, double-blind, placebo-controlled, fixed-dose studies (at doses of 50 mg per day to 400 mg per day) in adult outpatients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for major depressive disorder. In the first study, patients received 100 mg (n=114), 200 mg (n=116), or 400 mg (n=113) of PRISTIQ once daily, or placebo (n=118). In a second study, patients received either 200 mg (n=121) or 400 mg (n=124) of PRISTIQ once daily, or placebo (n=124). In two additional studies, patients received 50 mg (n=150 and n=164) or 100 mg (n=147 and n=158) of PRISTIQ once daily, or placebo (n=150 and n=161).
PRISTIQ showed superiority over placebo as measured by improvement in the 17-item Hamilton Rating Scale for Depression (HAM-D 17) total score in four studies and overall improvement, as measured by the Clinical Global Impressions Scale – Improvement (CGI-I), in three of the four studies. In studies directly comparing 50 mg per day and 100 mg per day there was no suggestion of a greater effect with the higher dose and adverse reactions and discontinuations were more frequent at higher doses [see Dosage and Administration (2.1)].
Table 9. Primary Efficacy (HAM-D17) Results for Short-term Studies:
| PRISTIQ | ||||||
|---|---|---|---|---|---|---|
| Study No. | Primary Endpoint: HAM-D17 | Placebo | 50 mg/day | 100 mg/day | 200 mg/day | 400 mg/day |
| 1 | Baseline Score (SD ?footnote?) | 23.1 (2.5) | 23.2 (2.5) | 22.9 (2.4) | 23.0 (2.2) | |
| Difference from Placebo (95% CI ?footnote?) | -2.9 ?footnote? (-5.1, -0.8) | -2.0 | -3.1 ?footnoteRef? (-5.2, -0.9) | |||
| 2 | Baseline Score (SD ?footnoteRef?) | 25.3 (3.3) | 24.8 (2.9) | 25.2 (3.2) | ||
| Difference from Placebo (95% CI ?footnoteRef?) | -3.3 ?footnoteRef? (-5.3, -1.2) | -2.8 ?footnoteRef? (-4.8, -0.7) | ||||
| 3 | Baseline Score (SD ?footnoteRef?) | 23.0 (2.6) | 23.4 (2.6) | 23.4 (2.6) | ||
| Difference from Placebo (95% CI ?footnoteRef?) | -1.9 ?footnoteRef? (-3.5, -0.3) | -1.5 | ||||
| 4 | Baseline Score (SD ?footnoteRef?) | 24.3 (2.6) | 24.3 (2.4) | 24.4 (2.7) | ||
| Difference from Placebo (95% CI ?footnoteRef?) | -2.5 ?footnoteRef? (-4.1, -0.9) | -3.0 ?footnoteRef? (-4.7, -1.4) | ||||
Analyses of the relationships between treatment outcome and age and treatment outcome and gender did not suggest any differential responsiveness on the basis of these patient characteristics. There was insufficient information to determine the effect of race on outcome in these studies.
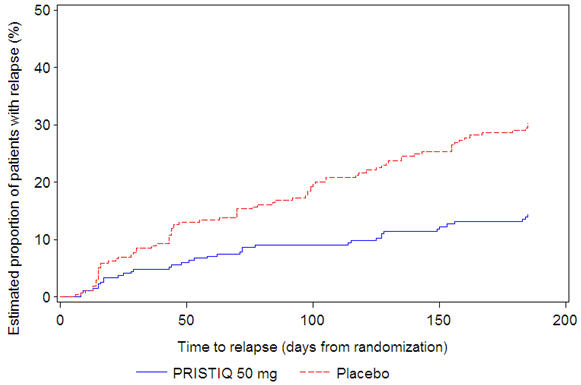
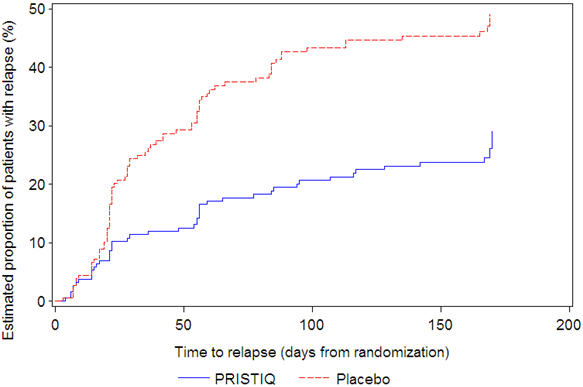
In a longer-term trial (Study 5), adult outpatients meeting DSM-IV criteria for major depressive disorder, who responded to 8 weeks of open-label acute treatment with 50 mg per day desvenlafaxine and subsequently remained stable for 12 weeks on desvenlafaxine, were assigned randomly in a double-blind manner to remain on active treatment or switch to placebo for up to 26 weeks of observation for relapse. Response during the open-label phase was defined as a HAM-D17 total score of ≤11 and CGI-I ≤2 at the day 56 evaluation; stability was defined as HAM-D17 total score of ≤11 and CGI-I ≤2 at week 20 and not having a HAM-D17 total score of ≥16 or a CGI-I score ≥4 at any office visit. Relapse during the double-blind phase was defined as follows: (1) a HAM-D17 total score of ≥16 at any office visit, (2) discontinuation for unsatisfactory efficacy response, (3) hospitalized for depression, (4) suicide attempt, or (5) suicide. Patients receiving continued desvenlafaxine treatment experienced statistically significantly longer time to relapse compared with placebo. At 26 weeks, the Kaplan-Meier estimated proportion of relapse was 14% with desvenlafaxine treatment versus 30% with placebo.
Figure 4. Estimated Proportion of Relapses vs. Number of Days since Randomization (Study 5):
In another longer-term trial (Study 6), adult outpatients meeting DSM-IV criteria for major depressive disorder and who responded to 12 weeks of acute treatment with desvenlafaxine were assigned randomly to the same dose (200 or 400 mg per day) they had received during acute treatment or to placebo for up to 26 weeks of observation for relapse. Response during the open-label phase was defined as a HAM-D17 total score of ≤ 11 at the day 84 evaluation. Relapse during the double-blind phase was defined as follows: (1) a HAM-D17 total score of ≥16 at any office visit, (2) a CGI-I score of ≥6 (versus day 84) at any office visit, or (3) discontinuation from the trial due to unsatisfactory response. Patients receiving continued desvenlafaxine treatment experienced statistically significantly longer time to relapse over the subsequent 26 weeks compared with those receiving placebo. At 26 weeks, the Kaplan-Meier estimated proportion of relapse was 29% with desvenlafaxine treatment versus 49% with placebo.
Figure 5. Estimated Proportion of Relapses vs. Number of Days since Randomization (Study 6):
In a postmarketing study, the efficacy of PRISTIQ at a dose lower than 50 mg per day was evaluated in an 8-week, multicenter, randomized, double-blind, placebo-controlled, fixed-dose study in adult outpatients with Major Depressive Disorder. The treatment arms were 25 mg (n=232), 50 mg (n=236), and placebo (n=231). The 50 mg dose was superior to placebo, as measured by the mean change from baseline on the HAMD-17. The 25 mg dose was not superior to placebo.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.