PROCAINAMIDE HCI Solution for injection Ref.[108396] Active ingredients: Procainamide
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
Procainamide Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of procainamide hydrochloride in water for injection. Each milliliter of the 2 mL vial contains procainamide hydrochloride 500 mg; methylparaben 1 mg and sodium metabisulfite 1.8 mg added in water for injection. Each milliliter of the 10 mL vial contains procainamide hydrochloride 100 mg; methylparaben 1 mg and sodium metabisulfite 0.8 mg added in water for injection. In both formulations, the solution may contain hydrochloric acid and/or sodium hydroxide for pH adjustment. pH 5.0 (4.0 to 6.0). Headspace nitrogen gassed.
Procainamide Hydrochloride Injection is intended for intravenous or intramuscular administration.
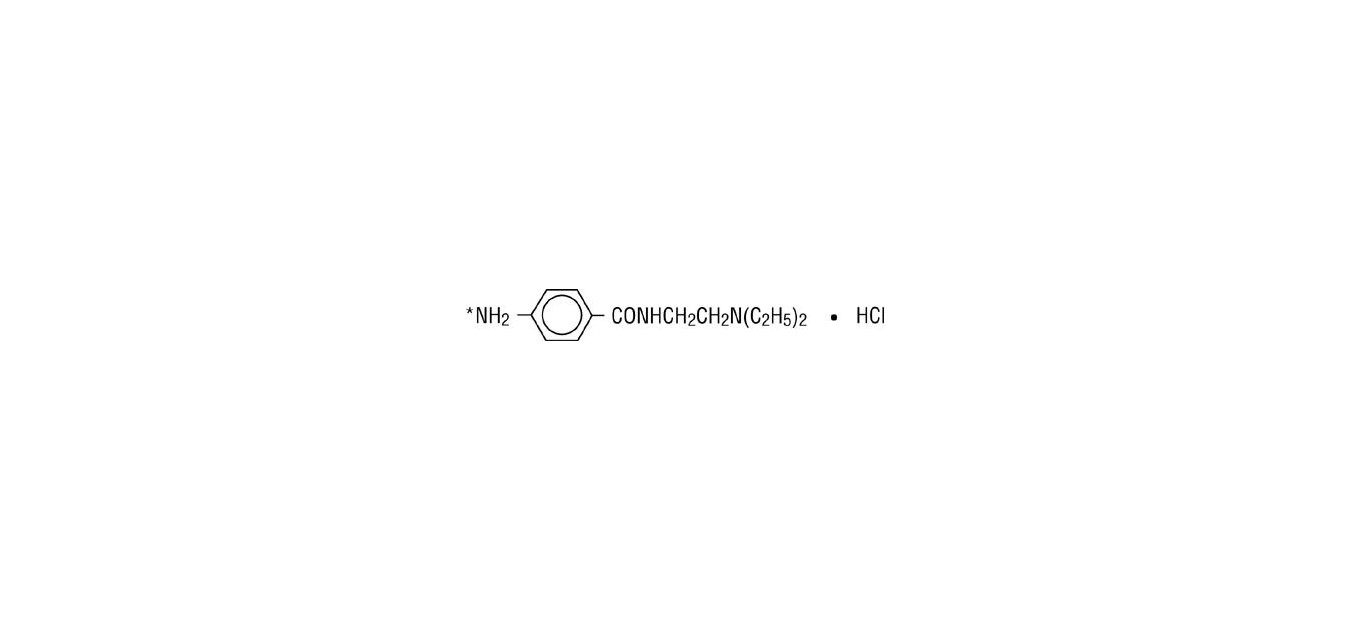
Procainamide hydrochloride, a Group 1A cardiac antiarrhythmic drug, is ρ-amino-N-[2-(diethylamino) ethyl] benzamide mono-hydrochloride. It has the following structural formula:
M.W. 271.79
* (locus for acetylation to N-acetyl procainamide).
It differs from procaine which is the p-aminobenzoyl ester of 2-(diethylamino)-ethanol. Procainamide as the free base has a pKa of 9.23; the monohydrochloride is very soluble in water.
| How Supplied | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
PROCAINAMIDE HCI INJECTION, USP is supplied in the following dosage forms. NDC 51662-1334-1 PROCAINAMIDE HCI INJECTION, USP 1gram/10mL TOTAL (100mg/mL) 10mL VIAL NDC 51662-1334-2 PROCAINAMIDE HCI INJECTION, USP 1gram/10mL TOTAL (100mg/mL) 10mL VIAL (1 vial per pouch) NDC 51662-1334-3 PROCAINAMIDE HCI INJECTION, USP 1gram/10mL TOTAL (100mg/mL) 10mL VIAL (1 vial per pouch, 25 pouches/box) HF Acquisition Co LLC, DBA HealthFirst, Mukilteo, WA 98275 Also supplied in the following manufacture supplied dosage forms Procainamide Hydrochloride Injection, USP is available in multiple-dose 10 mL vials providing 100 mg procainamide hydrochloride per mL and 2 mL vials providing 500 mg procainamide hydrochloride per mL.
The solutions, which are clear and colorless initially, may develop a slightly yellow color in time. This does not indicate a change which should preclude its use, but a solution any darker than light amber or otherwise discolored should not be used. Distributed by: Hospira Inc., Lake Forest, IL 60045 USA |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.