PYTEST Capsule (14C-Urea Breath Test) Ref.[11131] Active ingredients: Urea
Source: FDA, National Drug Code (US) Revision Year: 2020
2. Clinical Pharmacology
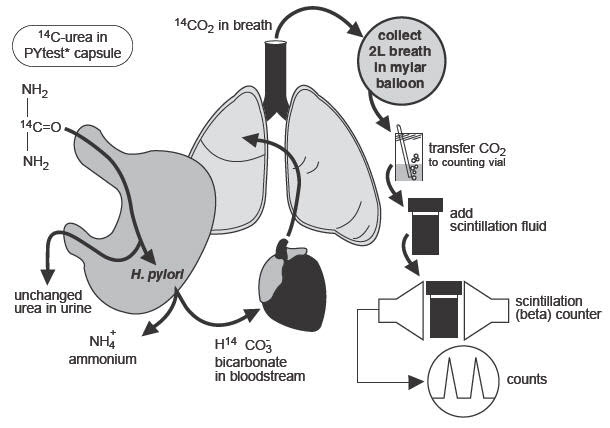
The urease enzyme is not present in mammalian cells, so the presence of urease in the stomach is evidence that bacteria are present. The presence of urease is not specific for H.pylori, but other bacteria are not usually found in the stomach. The principle of the breath test is shown in Figure 1.
Figure 1. Principle of Breath Test:
To detect H.pylori, urea labeled with 14C is swallowed by the patient. If gastric urease from H.pylori is present, urea is split to form CO2 and NH3 at the interface between the gastric epithelium and lumen and 14CO2 is absorbed into the blood and exhaled in the breath.
Following ingestion of the capsule by a patient with H.pylori, 14CO2excretion in the breath peaks between 10 and 15 minutes and declines thereafter with a biological half-life of about 15 minutes. 14C-urea that is not hydrolyzed by H.pylori is excreted in the urine with a half-life of approximately 12 hours. About 10% of the 14C remains in the body at 72 hours and is gradually excreted with a biological half-life of 40 days.
6.6. Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted with 14C-urea to evaluate its potential for carcinogenicity, impairment of fertility, or mutagenicity.
13. Clinical Studies
Two studies were performed. In both studies, patients with gastrointestinal symptoms underwent the breath test and an endoscopy. During the endoscopy, biopsy samples were taken from the antral gastric mucosa for histological analysis (2 samples, Giemsa stain) and rapid urease test (1 sample, CLOtest1). Breath samples were mailed to the TRI-MED lab where they were read in a liquid scintillation counter.
Results were reported as disintegrations per minute (DPM). Analysis for accuracy used the ten minute breath sample. A breath sample DPM <50 was defined as a negative result. DPM ≥200 was defined as a positive result. DPM in the range of 50-199 was classified as indeterminate.
STUDY 1
Of 186 patients who had histopathology and CLOtest1 (80 men, 106 women), 53 were infected with H.pylori as determined by agreement between histology and CLOtest1. The study results are summarized below:
Table 1. Study #1 (n=186, Indeterminate results included):
| Histology and CLOtest1 | |||||
|---|---|---|---|---|---|
| H.pylori | Positive | Negative | Total | ||
| PYtest1 (DPM 10m.) | Positive | 51 | 8 | 59 | ppv. 86% |
| Indeterminate | 1 | 8 | 9 | ||
| Negative | 1 | 117 | 118 | npv. 99% | |
| Total | 53 | 133 | 186 | ||
| sensitivity 96% | specificity 88% | ||||
Notes: PYtest1 at 10 min. was compared to the gold standard of biopsy results in which histology and CLOtest1 concurred. Patients who did not have both biopsy tests performed, or in whom the tests differed, were excluded from analysis. There was no statistical difference in test accuracy based on gender of patient.
ppv = positive predictive value (true positive divided by total
PYtest1 positive)
npv = negative predictive value (true negative divided by total
PYtest1 negative)
STUDY 2
Breath tests were performed on 436 outpatients attending gastroenterology practices at sites in the United States. Seventy-six patients (40 men, 36 women) who had histology and CLOtest1 were evaluated. The results are summarized below:
Table 2. Study #2 (n=76, Indeterminate results included):
| Histology and CLOtest1 | |||||
|---|---|---|---|---|---|
| H.pylori | Positive | Negative | Total | ||
| PYtest1 (DPM 10m.) | Positive | 22 | 0 | 22 | ppv. 100% |
| Indeterminate | 4 | 2 | 6 | ||
| Negative | 1 | 47 | 48 | npv. 98% | |
| Total | 27 | 49 | 76 | ||
| sensitivity 82% | specificity 96% | ||||
Notes: PYtest1 at 10 min. was compared to the gold standard of biopsy results in which histology and CLOtest concurred. Patients who did not have both biopsy tests performed, or in whom the tests differed, were excluded from analysis. There was no statistical difference in test accuracy based on gender of patient.
ppv = positive predictive value (true positive divided by total
PYtest1 positive)
npv = negative predictive value (true negative divided by total
PYtest1 negative)
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.