QDENGA Powder and solvent for solution for injection Ref.[50598] Active ingredients: Dengue vaccine
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Takeda GmbH, Byk-Gulden-Str. 2, 78467 Konstanz, Germany
4.1. Therapeutic indications
Qdenga is indicated for the prevention of dengue disease in individuals from 4 years of age.
The use of Qdenga should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Individuals from 4 years of age
Qdenga should be administered as a 0.5 mL dose at a two-dose (0 and 3 months) schedule.
The need for a booster dose has not been established.
Other paediatric population (children <4 years of age)
The safety and efficacy of Qdenga in children aged less than 4 years has not yet been established. Currently available data are described in section 4.8 but no recommendation on a posology can be made.
Elderly
No dose adjustment is required in elderly individuals ≥60 years of age. See section 4.4.
Method of administration
After complete reconstitution of the lyophilised vaccine with the solvent, Qdenga should be administered by subcutaneous injection preferably in the upper arm in the region of deltoid.
Qdenga must not be injected intravascularly, intradermally or intramuscularly.
The vaccine should not be mixed in the same syringe with any vaccines or other parenteral medicinal products.
For instructions on reconstitution of Qdenga before administration, see section 6.6.
4.9. Overdose
No cases of overdose have been reported.
6.3. Shelf life
18 months.
After reconstitution with the solvent provided, Qdenga should be used immediately.
If not used immediately, Qdenga must be used within 2 hours.
Chemical and physical in-use stability have been demonstrated for 2 hours at room temperature (up to 32.5°C) from the time of reconstitution of the vaccine vial. After this time period, the vaccine must be discarded. Do not return it to the refrigerator.
From a microbiological point of view Qdenga should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4. Special precautions for storage
Store in a refrigerator (2°C to 8°C). Do not freeze.
Store in the original package.
For storage conditions after reconstitution of Qdenga, see section 6.3.
6.5. Nature and contents of container
Qdenga powder and solvent for solution for injection:
- Powder (1 dose) in glass vial (Type-I glass), with a stopper (butyl rubber) and aluminium seal with green flip-off plastic cap + 0.5 mL solvent (1 dose) in glass vial (Type-I glass), with a stopper (bromobutyl rubber) and aluminium seal with purple flip-off plastic cap
Pack size of 1 or 10.
Qdenga powder and solvent for solution for injection in pre-filled syringe:
- Powder (1 dose) in vial (Type-I glass), with a stopper (butyl rubber) and aluminium seal with green flip-off plastic cap + 0.5 mL solvent (1 dose) in pre-filled syringe (Type-I glass), with a plunger stopper (bromobutyl) and a tip cap (polypropylene), with 2 separate needles
Pack size of 1 or 5.
- Powder (1 dose) in vial (Type-I glass), with a stopper (butyl rubber) and aluminium seal with green flip-off plastic cap + 0.5 mL solvent (1 dose) in pre-filled syringe (Type-I glass), with a plunger stopper (bromobutyl) and a tip cap (polypropylene), without needles
Pack size of 1 or 5.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Instructions for reconstitution of the vaccine with the solvent presented in vial
Qdenga is a 2-component vaccine that consists of a vial containing lyophilised vaccine and a vial containing solvent. The lyophilised vaccine must be reconstituted with solvent prior to administration.
Use only sterile syringes for reconstitution and injection of Qdenga. Qdenga should not be mixed with other vaccines in the same syringe.
To reconstitute Qdenga, use only the solvent (0.22% sodium chloride solution) supplied with the vaccine since it is free of preservatives or other anti-viral substances. Contact with preservatives, antiseptics, detergents, and other anti-viral substances is to be avoided since they may inactivate the vaccine.
Remove the vaccine and solvent vials from the refrigerator and place at room temperature for approximately 15 minutes.
Solvent vial:
- Remove the caps from both vials and clean the surface of stoppers on top of the vials using an alcohol wipe.
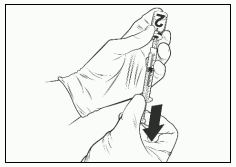
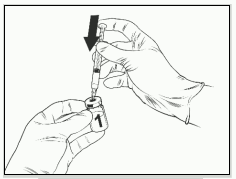
- Attach a sterile needle to a sterile 1 mL syringe and insert the needle into the solvent vial. The recommended needle is 23G.
- Slowly press the plunger completely down.
- Turn the vial upside down, withdraw the entire contents of the vial and continue to pull plunger out to 0.75 mL. A bubble should be seen inside of the syringe.
- Invert the syringe to bring the bubble back to the plunger
Lyophilised vaccine vial:
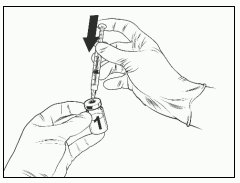
- Insert the needle of the syringe assembly into the lyophilised vaccine vial.
- Direct the flow of the solvent toward the side of the vial while slowly depressing the plunger to reduce the chance of forming bubbles.
Reconstituted vaccine:
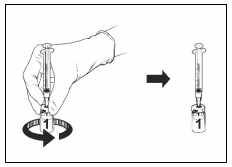
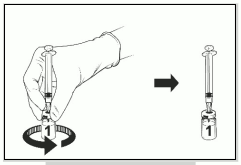
- Release your finger from the plunger and, holding the assembly on a flat surface, gently swirl the vial in both directions with the needle syringe assembly attached.
- DO NOT SHAKE. Foam and bubbles may form in the reconstituted product.
- Let the vial and syringe assembly sit for a while until the solution becomes clear. This takes about 30-60 seconds.
Following reconstitution, the resulting solution should be clear, colourless to pale yellow, and essentially free of foreign particulates. Discard the vaccine if particulates are present and/or if it appears discoloured.
Reconstituted vaccine:
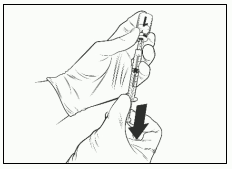
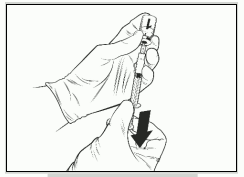
- Withdraw the entire volume of the reconstituted Qdenga solution with the same syringe until an air bubble appears in the syringe.
- Remove the needle syringe assembly from the vial.
- Hold the syringe with the needle pointing upwards, tap the side of the syringe to bring the air bubble to the top, discard the attached needle and replace with a new sterile needle, expel the air bubble until a small drop of the liquid forms at the top of the needle. The recommended needle is 25G 16 mm.
- Qdenga is ready to be administered by subcutaneous injection.
Qdenga should be administered immediately after reconstitution. Chemical and physical in-use stability have been demonstrated for 2 hours at room temperature (up to 32.5°C) from the time of reconstitution of the vaccine vial. After this time period, the vaccine must be discarded. Do not return it to the refrigerator. From a microbiological point of view Qdenga should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
Instructions for reconstitution of the vaccine with solvent presented in pre-filled syringe
Qdenga is a 2-component vaccine that consists of a vial containing lyophilised vaccine and solvent provided in the pre-filled syringe. The lyophilised vaccine must be reconstituted with solvent prior to administration.
Qdenga should not be mixed with other vaccines in the same syringe.
To reconstitute Qdenga, use only the solvent (0.22% sodium chloride solution) in the pre-filled syringe supplied with the vaccine since it is free of preservatives or other anti-viral substances. Contact with preservatives, antiseptics, detergents, and other anti-viral substances is to be avoided since they may inactivate the vaccine.
Remove the vaccine vial and pre-filled syringe solvent from the refrigerator and place at room temperature for approximately 15 minutes.
Lyophilised vaccine vial:
- Remove the cap from the vaccine vial and clean the surface of stopper on top of the vial using an alcohol wipe.
- Attach a sterile needle to the pre-filled syringe and insert the needle into the vaccine vial. The recommended needle is 23G.
- Direct the flow of the solvent toward the side of the vial while slowly depressing the plunger to reduce the chance of forming bubbles.
Reconstituted vaccine:
- Release your finger from the plunger and, holding the assembly on a flat surface, gently swirl the vial in both directions with the needle syringe assembly attached.
- DO NOT SHAKE. Foam and bubbles may form in the reconstituted product.
- Let the vial and syringe assembly sit for a while until the solution becomes clear. This takes about 30-60 seconds.
Following reconstitution, the resulting solution should be clear, colourless to pale yellow, and essentially free of foreign particulates. Discard the vaccine if particulates are present and/or if it appears discoloured.
Reconstituted vaccine:
- Withdraw the entire volume of the reconstituted Qdenga solution with the same syringe until an air bubble appears in the syringe.
- Remove the needle syringe assembly from the vial. Hold the syringe with the needle pointing upwards, tap the side of the syringe to bring the air bubble to the top, discard the attached needle and replace with a new sterile needle, expel the air bubble until a small drop of the liquid forms at the top of the needle. The recommended needle is 25G 16 mm.
- Qdenga is ready to be administered by subcutaneous injection.
Qdenga should be administered immediately after reconstitution. Chemical and physical in-use stability have been demonstrated for 2 hours at room temperature (up to 32.5°C) from the time of reconstitution of the vaccine vial. After this time period, the vaccine must be discarded. Do not return it to the refrigerator. From a microbiological point of view Qdenga should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.