QFITLIA Solution for injection Ref.[115543] Active ingredients: Fitusiran
Source: FDA, National Drug Code (US) Revision Year: 2025
Product description
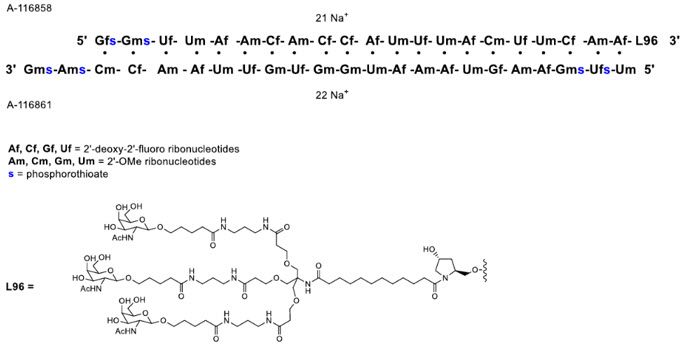
QFITLIA injection contains fitusiran, an antithrombin-directed double-stranded small interfering ribonucleic acid (siRNA), which is covalently linked to a ligand containing a triantennary N-acetylgalactosamine (GalNAc) moiety. The molecular formula of fitusiran sodium is C520H636F21N175Na43O309P43S6 and the molecular weight is 17,193 Da. Fitusiran drug substance is a white to pale yellow powder and freely soluble in water and phosphate buffered saline. The pH of a 1% aqueous solution of fitusiran drug substance in 50 mM KCl is 5.4 (4.4–7.3). It has the following structural formula:
QFITLIA is supplied as a sterile, preservative-free, clear, colorless to pale yellow solution for subcutaneous administration.
Each 50 mg single-dose prefilled pen delivers 50 mg fitusiran (equivalent to 53.0 mg fitusiran sodium) in 0.5 mL, and also contains dibasic sodium phosphate (0.585 mg), monobasic sodium phosphate (0.044 mg), sodium chloride (2.455 mg), and Water for Injection, USP. Phosphoric acid (concentrated) and sodium hydroxide may be added to adjust to pH 7.0.
Each 20 mg single-dose vial delivers 20 mg fitusiran (equivalent to 21.2 mg fitusiran sodium) in 0.2 mL, and also contains dibasic sodium phosphate (0.234 mg), monobasic sodium phosphate (0.018 mg), sodium chloride (0.982 mg), and Water for Injection, USP. Phosphoric acid (concentrated) and sodium hydroxide may be added to adjust to pH 7.0.
| Dosage Forms and Strengths |
|---|
|
QFITLIA is a clear, colorless to pale yellow solution in a single-dose:
|
| How Supplied |
|---|
|
QFITLIA (fitusiran) is a clear, colorless to pale yellow solution supplied in a single-dose prefilled pen or a single-dose vial. Each prefilled pen is designed to deliver 50 mg of QFITLIA in 0.5 mL (NDC 58468-0348-1). Each vial is designed to deliver 20 mg of QFITLIA in 0.2 mL (NDC 58468-0347-1). QFITLIA is available in cartons containing 1 prefilled pen or 1 vial. Manufactured by: Genzyme Corporation, Cambridge, MA 02141 |
Drugs
| Drug | Countries | |
|---|---|---|
| QFITLIA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.