RABIPUR Powder and solvent for solution for injection Ref.[27698] Active ingredients: Rabies vaccine
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2021 Publisher: Bavarian Nordic A/S, Philip Heymans Allé 3, 2900 Hellerup, Denmark
4.1. Therapeutic indications
Rabipur is indicated for active immunization against rabies in individuals of all ages. See Sections 4.2 and 5.1 for detailed information about pre- and post-exposure prophylaxis.
Rabipur should be used in accordance with official recommendations.
4.2. Posology and method of administration
Posology
The recommended dose for both primary immunization and boosters is 1.0 ml.
Pre-exposure prophylaxis
Primary immunization
In previously unvaccinated individuals, three doses administered according to the conventional or rapid regimen, as shown in Table 1.
Table 1. Primary immunization regimens:
| Conventional regimen | Rapid Regimen* | |
|---|---|---|
| 1st dose | Day 0 | Day 0 |
| 2nd dose | Day 7 | Day 3 |
| 3rd dose | Day 21 (or 28) | Day 7 |
* The rapid regimen should only be considered for adults aged 18-65 years not able to complete the conventional pre-exposure prophylaxis regimen within 21 or 28 days before protection is required.
Booster doses
Booster doses are generally recommended every 2-5 years. Timing for booster after vaccination with with rapid regimen has not yet been established (see also section 5.1). Serological testing for the presence of antibody ≥ 0.5 IU/ml to assess the need for booster doses should be conducted in accordance with official recommendations.
Rabipur may be used to boost individuals previously immunized with any human diploid cell rabies vaccine.
Post-exposure prophylaxis
Post-exposure prophylaxis should commence as soon as possible after exposure.
Table 2 summarises recommendations for post-exposure prophylaxis, including immunization, according to the type of exposure.
Table 2. Recommended post-exposure prophylaxis according to type of exposure:
| Category of exposure | Type of exposure to a domestic or wild a) animal suspected or confirmed to be rabid, or animal unavailable for testing | Recommended post-exposure prophylaxis |
|---|---|---|
| I | Touching or feeding animals Licks on intact skin Contact of intact skin with secretions or excretions of a rabid animal or human case | None, if reliable case history is available. |
| II | Nibbling of uncovered skin Minor scratches or abrasions without bleeding | Administer vaccine immediatelyb Stop treatment if animal remains healthy throughout an observation period of 10 daysc or is proven to be negative for rabies by a reliable laboratory using appropriate diagnostic techniques. |
| III | Single or multiple transdermal bitesd or scratches, licks on broken skin. Contamination of mucous membrane with saliva (i.e. licks). Exposure to batse. | Administer rabies vaccine immediately, and rabies immunoglobulin, preferably as soon as possible after initiation of post-exposure prophylaxis. Rabies immunoglobulin can be injected up to 7 days after first vaccine dose administration. Stop treatment if animal remains healthy throughout an observation period of 10 days or is proven to be negative for rabies by reliable laboratory using appropriate diagnostic techniques. |
a Exposure to rodents, rabbits or hares does not routinely require rabies post-exposure prophylaxis.
b If an apparently healthy dog or cat in, or from a low-risk area is placed under observation, treatment may be delayed.
c This observation period applies only to dogs and cats. Except for threatened or endangered species, other domestic and wild animals suspected of being rabid should be euthanized and their tissues examined for the presence of rabies antigen by appropriate laboratory techniques.
d Bites especially on the head, neck, face, hands and genitals are category III exposures because of the rich innervation of these areas.
e Post-exposure prophylaxis should be considered when contact between a human and a bat has occurred, unless the exposed person can rule out a bite or scratch or exposure of a mucous membrane.
In-post-exposure prophylaxis of previously unvaccinated individuals, the vaccine should be administered according to Table 3.
Table 3. Post-exposure immunization regimens for previously unvaccinated individuals:
| Essen regimen (5 doses) | Zagreb regimen (4 doses) | Reduced Essen regimen (4 doses)2 | |
|---|---|---|---|
| 1st dose | Day 0 | Day 0, 2 doses1 | Day 0 |
| 2nd dose | Day 3 | Day 3 | |
| 3rd dose | Day 7 | Day 7 | Day 7 |
| 4th dose | Day 14 | Day 21 | Day 14 |
| 5th dose | Day 28 |
1 one injection in each of the two deltoids or thigh sites
2 this shortened Essen regimen may be used as an alternative for healthy, immunocompetent individuals provided they receive wound care plus rabies immunoglobulin in category III as well as in category II exposures and a WHO-prequalified rabies vaccine
In previously vaccinated individuals, post-exposure prophylaxis consists of two doses administered on days 0 and 3. Rabies immunoglobulin is not indicated in such cases.
In immunocompromised individuals with category II and III exposures, 5 doses should be given in combination with comprehensive wound management and local infiltration of rabies immunoglobulin as shown in Table 4.
Table 4. Post-exposure immunization regimens for immunocompromised individuals:
| Essen regimen | Alternative to Essen | |
|---|---|---|
| 1st dose | Day 0 | Day 0, 2 doses1 |
| 2nd dose | Day 3 | Day 3 |
| 3rd dose | Day 7 | Day 7 |
| 4th dose | Day 14 | Day 14 |
| 5th dose | Day 28 | Day 28 |
1 Two doses of vaccine may be given on day 0, that is, a single dose of 1.0 ml vaccine should be injected into the right deltoid and another single dose into the left deltoid muscle. In small children, one dose should be given into the anterolateral region of each thigh. This would result in a total of 6 doses.
When feasible, the rabies virus neutralising antibody response should be measured 2 to 4 weeks (preferably on day 14) following the start of vaccination to assess the possible need for an additional dose of the vaccine. Immunosuppressive agents should not be administered during postexposure therapy unless essential for the treatment of other conditions (see section 4.5).
Paediatric population
Paediatric individuals receive the same dose as adults (1.0 ml).
Method of administration
Rabipur is for intramuscular administration only. For adults and children ≥ 2 years of age, the vaccine should be administered into the deltoid muscle. For children < 2 years, the anterolateral area of the thigh is recommended.
For instructions on reconstitution of the vaccine before administration, see section 6.6.
4.9. Overdose
No symptoms of overdose are known.
6.3. Shelf life
48 months.
After reconstitution the vaccine is to be used immediately.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C). Do not freeze.
Keep the vial and the syringe in the outer carton in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3
The vaccine may not be used after the expiration date given on package and container.
6.5. Nature and contents of container
Package with:
1 vial (type I glass) of freeze-dried vaccine with stopper (chlorobutyl)
1 disposable pre-filled syringe (type I glass) of Sterile Diluent for reconstitution (1 mL) with plunger-stopper (bromobutyl) without needle and with a tip-cap (bromobutyl). 1 small orange needle for injection (25 gauge, 25 mm) and one long green needle for reconstitution (21 gauge, 40 mm)
6.6. Special precautions for disposal and other handling
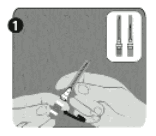
Instruction for Use of Rabipur disposable pre-filled syringe:
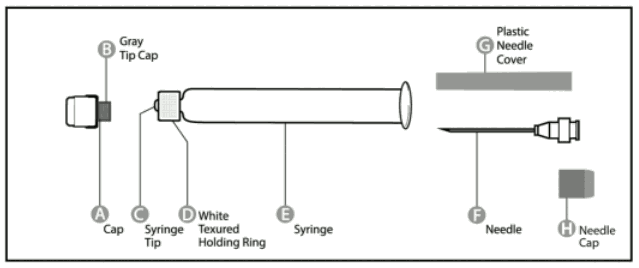
Pre-filled syringe
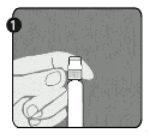
Step 1: With one hand, hold the syringe (E) with the cap pointing upward. Be sure to hold the syringe by the white textured holding ring (D).
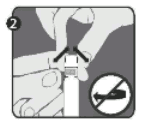
Step 2: With the other hand, grasp the cap (A) and firmly rock it back and forth to break its connection to the holding ring (D). Do not twist or turn the cap.
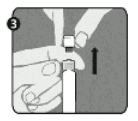
Step 3: Lift up to remove the cap (A) and the attached gray tip cap (B). Be careful not to touch the sterile syringe tip ©.
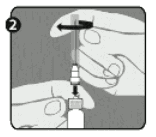
<uNeedle application (these instructions apply to both the green and the orange needles):
Step 1: Twist to remove the cap (H) from the green reconstitution needle. Do not remove the plastic cover (G). This needle is the longer of the two needles.
Step 2: With one hand, firmly hold syringe (E) by white textured holding ring (D). With your other hand, insert needle (F) and twist clockwise until it locks into place. Once needle is locked, remove its plastic cover (G).
The syringe is now ready for use.
Instructions for reconstituting Rabipur with the use of pre-filled syringe:
The vaccine should be visually inspected both before and after reconstitution for any foreign particulate matter and or change in physical appearance. The vaccine must not be used if any change in the appearance of the vaccine has taken place.
The reconstituted vaccine is clear to slightly opalescent and colourless to slightly pink.
The powder for solution should be reconstituted using the solvent for solution supplied and carefully agitated prior to injection. The reconstituted vaccine should be used immediately.
During manufacturing, the vial is sealed under vacuum. Therefore to prevent problems in withdrawing the reconstituted vaccine from the vial after reconstitution of the vaccine, it is recommended to unscrew the syringe from the needle to eliminate the negative pressure. After that, the vaccine can be easily withdrawn from the vial. It is not recommended to induce excess pressure, since over-pressurization will create the problems in withdrawing the proper amount of the vaccine.
After completing the reconstitution of the vaccine, remove the cap from the orange administration needle (as explained in step 1 for the green needle) and replace the green reconstitution needle with the orange administration needle or other appropriate needle.
Any unused vaccine or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.