RANIVISIO Solution for injection Ref.[50220] Active ingredients: Ranibizumab
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Midas Pharma GmbH, Rheinstraße 49, D-55218 Ingelheim, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, antineovascularisation agents
ATC code: S01LA04
Ranivisio is a biosimilar medicinal product.
Mechanism of action
Ranibizumab is a humanised recombinant monoclonal antibody fragment targeted against human vascular endothelial growth factor A (VEGF-A). It binds with high affinity to the VEGF-A isoforms (e.g. VEGF110, VEGF121 and VEGF165), thereby preventing binding of VEGF-A to its receptors VEGFR-1 and VEGFR-2. Binding of VEGF-A to its receptors leads to endothelial cell proliferation and neovascularisation, as well as vascular leakage, all of which are thought to contribute to the progression of the neovascular form of age-related macular degeneration, pathologic myopia and CNV or to visual impairment caused by either diabetic macular oedema or macular oedema secondary to RVO in adults.
Clinical efficacy and safety
Treatment of wet AMD
In wet AMD, the clinical safety and efficacy of ranibizumab have been assessed in three randomised, double-masked, sham- or active-controlled studies of 24 months duration in patients with neovascular AMD. A total of 1,323 patients (879 active and 444 control) were enrolled in these studies.
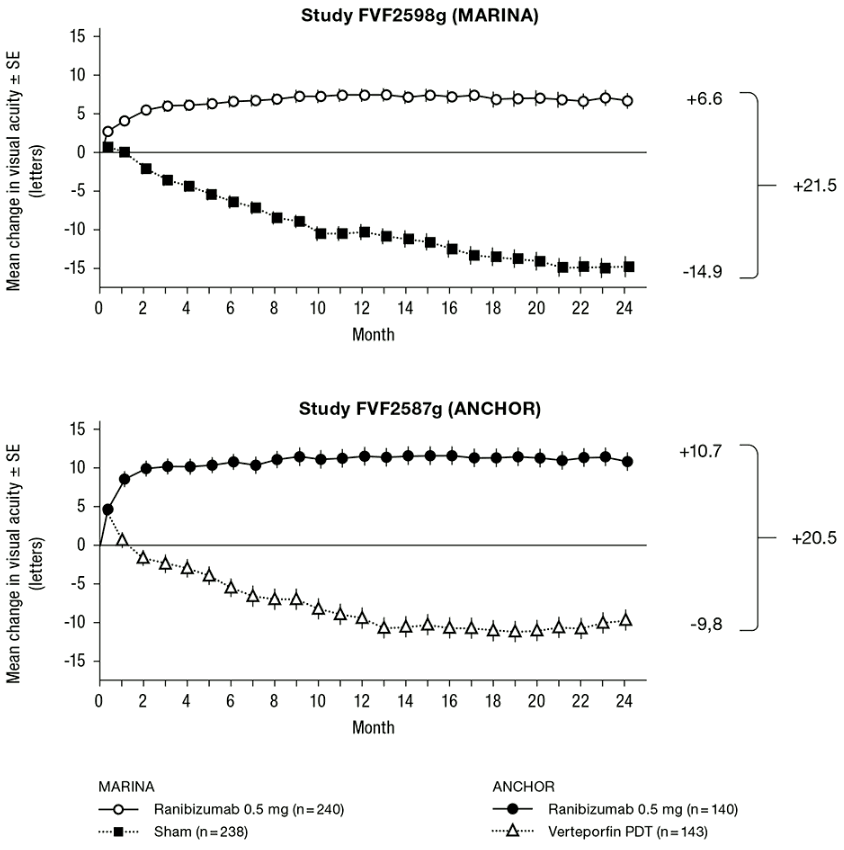
In study FVF2598g (MARINA), 716 patients with minimally classic or occult with no classic lesions were randomised in a 1:1:1 ratio to receive monthly injections of ranibizumab 0.3 mg, ranibizumab 0.5 mg or sham.
In study FVF2587g (ANCHOR), 423 patients with predominantly classic CNV lesions were randomised in a 1:1:1 ratio to receive ranibizumab 0.3 mg monthly, ranibizumab 0.5 mg monthly or verteporfin PDT (at baseline and every 3 months thereafter if fluorescein angiography showed persistence or recurrence of vascular leakage).
Key outcome measures are summarised in Table 1 and Figure 1.
Table 1. Outcomes at Month 12 and Month 24 in study FVF2598g (MARINA) and FVF2587g (ANCHOR):
| FVF2598g (MARINA) | FVF2587g (ANCHOR) | ||||
|---|---|---|---|---|---|
| Outcome measure | Month | Sham (n=238) | Ranibizumab 0.5 mg (n=240) | Verteporfin PDT (n=143) | Ranibizumab 0.5 mg (n=140) |
| Loss of <15 letters in visual acuity (%)a (maintenance of vision, primary endpoint) | Month 12 | 62% | 95% | 64% | 96% |
| Month 24 | 53% | 90% | 66% | 90% | |
| Gain of ≥15 letters in visual acuity (%)a | Month 12 | 5% | 34% | 6% | 40% |
| Month 24 | 4% | 33% | 6% | 41% | |
| Mean change in visual acuity (letters) (SD)a | Month 12 | -10.5 (16.6) | +7.2 (14.4) | -9.5 (16.4) | +11.3 (14.6) |
| Month 24 | -14.9 (18.7) | +6.6 (16.5) | -9.8 (17.6) | +10.7 (16.5) | |
a p<0.01
Figure 1. Mean change in visual acuity from baseline to Month 24 in study FVF2598g (MARINA) and study FVF2587g (ANCHOR):
Results from both trials indicated that continued ranibizumab treatment may also be of benefit in patients who lost ≥15 letters of best-corrected visual acuity (BCVA) in the first year of treatment.
Statistically significant patient-reported visual functioning benefits were observed in both MARINA and ANCHOR with ranibizumab treatment over the control group as measured by the NEI VFQ-25.
In study FVF3192g (PIER), 184 patients with all forms of neovascular AMD were randomised in a 1:1:1 ratio to receive ranibizumab 0.3 mg, ranibizumab 0.5 mg or sham injections once a month for 3 consecutive doses, followed by a dose administered once every 3 months. From Month 14 of the study, sham-treated patients were allowed to receive ranibizumab and from Month 19, more frequent treatments were possible. Patients treated with ranibizumab in PIER received a mean of 10 total treatments.
After an initial increase in visual acuity (following monthly dosing), on average, patients' visual acuity declined with quarterly dosing, returning to baseline at Month 12 and this effect was maintained in most ranibizumab-treated patients (82%) at Month 24. Limited data from sham subjects who later received ranibizumab suggested that early initiation of treatment may be associated with better preservation of visual acuity. Data from two studies (MONT BLANC, BPD952A2308 and DENALI, BPD952A2309) conducted post approval confirmed the efficacy of ranibizumab but did not demonstrate additional effect of the combined administration of verteporfin (Visudyne PDT) and ranibizumab compared to ranibizumab monotherapy.
Treatment of visual impairment due to CNV secondary to PM
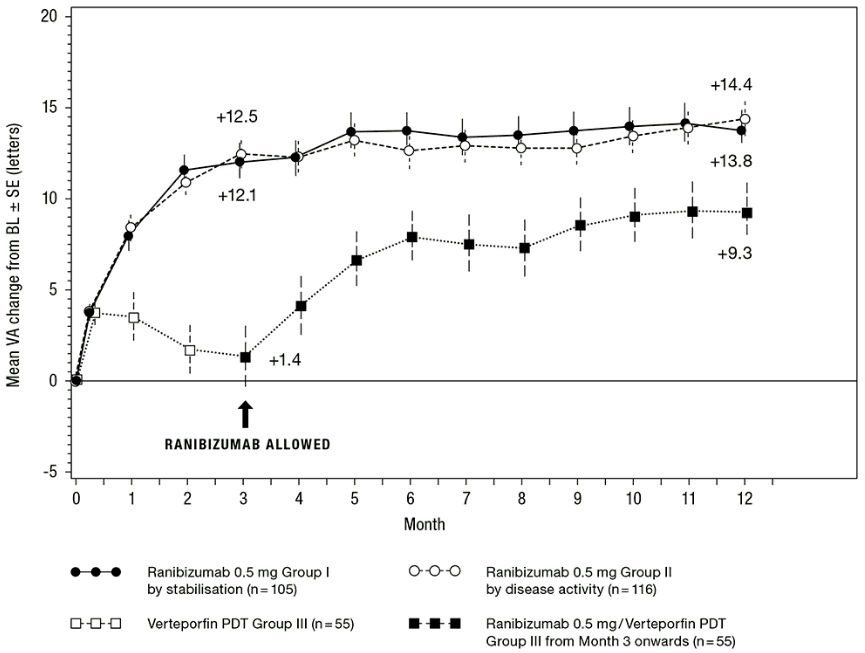
The clinical safety and efficacy of ranibizumab in patients with visual impairment due to CNV in PM have been assessed based on the 12-month data of the double-masked, controlled pivotal study F2301 (RADIANCE). In this study 277 patients were randomised in a 2:2:1 ratio to the following arms:
- Group I (ranibizumab 0.5 mg, dosing regimen driven by “stability” criteria defined as no change in BCVA compared to two preceding monthly evaluations).
- Group II (ranibizumab 0.5 mg, dosing regimen driven by “disease activity” criteria defined as vision impairment attributable to intra- or subretinal fluid or active leakage due to the CNV lesion as assessed by optical coherence tomography and/or fluorescence angiography).
- Group III (vPDT – patients were allowed to receive ranibizumab treatment as of Month 3).
In Group II, which is the recommended posology (see section 4.2), 50.9% of patients required 1 or 2 injections, 34.5% required 3 to 5 injections and 14.7% required 6 to 12 injections over the 12-month study period. 62.9% of Group II patients did not require injections in the second 6 months of the study.
The key outcomes from RADIANCE are summarised in Table 2 and Figure 2.
Table 2. Outcomes at Month 3 and 12 (RADIANCE):
| Group I Ranibizumab 0.5 mg “vision stability” (n=105) | Group II Ranibizumab 0.5 mg “disease activity” (n=116) | Group III vPDTb (n=55) | |
|---|---|---|---|
| Month 3 | |||
| Mean average BCVA change from Month 1 to Month 3 compared to baselinea (letters) | +10.5 | +10.6 | +2.2 |
| Proportion of patients who gained: ≥15 letters, or reached ≥84 letters in BCVA | 38.1% | 43.1% | 14.5% |
| Month 12 | |||
| Number of injections up to Month 12 | |||
| Mean Median | 4.6 4.0 | 3.5 2.5 | N/A N/A |
| Mean average BCVA change from Month 1 to Month 12 compared to baseline (letters) | +12.8 | +12.5 | N/A |
| Proportion of patients who gained | |||
| ≥15 letters, or reached ≥84 letters in BCVA | 53.3% | 51.7% | N/A |
a p<0.00001 comparison with vPDT control
b Comparative control up to Month 3. Patients randomised to vPDT were allowed to receive ranibizumab treatment as of Month 3 (in Group III, 38 patients received ranibizumab as of Month 3)
Figure 2. Mean change from baseline BCVA over time to Month 12 (RADIANCE):
The improvement of vision was accompanied by a reduction in central retinal thickness.
Patient-reported benefits were observed with ranibizumab treatment arms over vPDT (p-value <0.05) in terms of improvement in the composite score and several subscales (general vision, near activities, mental health and dependency) of the NEI VFQ-25.
Treatment of visual impairment due to CNV (other than secondary to PM and wet AMD)
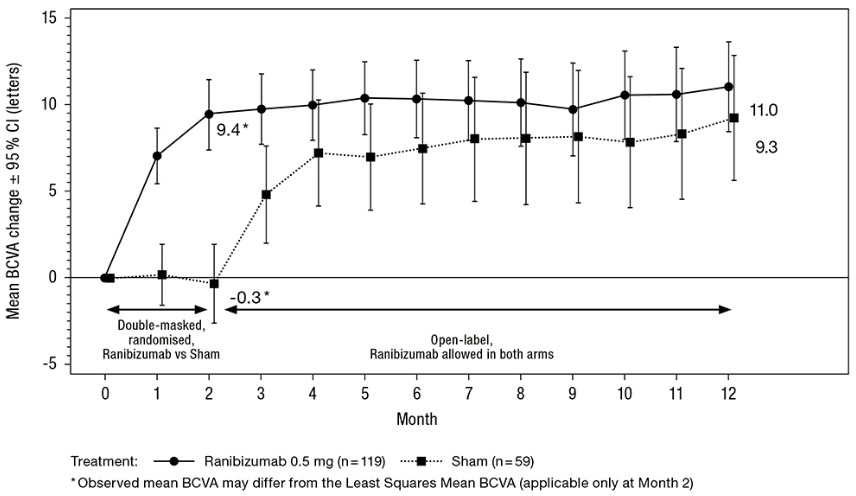
The clinical safety and efficacy of ranibizumab in patients with visual impairment due to CNV have been assessed based on the 12-month data of the double-masked, sham-controlled pivotal study G2301 (MINERVA). In this study 178 adult patients were randomised in a 2:1 ratio to receive:
- ranibizumab 0.5 mg at baseline, followed by an individualised dosing regimen driven by disease activity as assessed by visual acuity and/or anatomical parameters (e.g. VA impairment, intra/subretinal fluid, haemorrhage or leakage);
- sham injection at baseline, followed by an individualised treatment regimen driven by disease activity.
At Month 2, all patients received open-label treatment with ranibizumab as needed.
Key outcome measures from MINERVA are summarised in Table 3 and Figure 3. An improvement of vision was observed and was accompanied by a reduction in central subfield thickness over the 12-month period.
The mean number of injections given over 12 months was 5.8 in the ranibizumab arm versus 5.4 in those patients in the sham arm who were eligible to receive ranibizumab from Month 2 onwards. In the sham arm 7 out of 59 patients did not receive any treatment with ranibizumab in the study eye during the 12-month period.
Table 3. Outcomes at Month 2 (MINERVA):
| Ranibizumab 0.5 mg (n=119) | Sham (n=59) | |
|---|---|---|
| Mean BCVA change from baseline to Month 2a | 9.5 letters | -0.4 letters |
| Patients gaining ≥15 letters from baseline or reaching 84 letters at Month 2 | 31.4% | 12.3% |
| Patients not losing >15 letters from baseline at Month 2 | 99.2% | 94.7% |
| Reduction in CSFTb from baseline to Month 2a | 77 μm | -9.8 μm |
a One-sided p<0.001 comparison with sham control
b CSFT – central retinal subfield thickness
Figure 3. Mean change from baseline BCVA over time to Month 12 (MINERVA):
When comparing ranibizumab versus sham control at Month 2, a consistent treatment effect both overall and across baseline aetiology subgroups was observed:
Table 4. Treatment effect overall and across baseline aetiology subgroups:
| Overall and per baseline aetiology | Treatment effect over sham [letters] | Patient numbers [n] (treatment +sham) |
|---|---|---|
| Overall | 9.9 | 178 |
| Angioid streaks | 14.6 | 27 |
| Post-inflammatory retinochoroidopathy | 6.5 | 28 |
| Central serous chorioretinopathy | 5.0 | 23 |
| Idiopathic chorioretinopathy | 11.4 | 63 |
| Miscellaneous aetiologiesa | 10.6 | 37 |
a encompasses different aetiologies of low frequency of occurrence not included in the other subgroups
In the pivotal study G2301 (MINERVA), five adolescent patients aged 12 to 17 years with visual impairment secondary to CNV received open-label treatment with ranibizumab 0.5 mg at baseline followed by an individualised treatment regimen as for the adult population. BCVA improved from baseline to Month 12 in all five patients, ranging from 5 to 38 letters (mean of 16.6 letters). The improvement of vision was accompanied by a stabilisation or reduction in central subfield thickness over the 12-month period. The mean number of ranibizumab injections given in the study eye over 12 months was 3 (ranged from 2 to 5). Overall, ranibizumab treatment was well tolerated.
Treatment of visual impairment due to DME
The efficacy and safety of ranibizumab have been assessed in three randomised, controlled studies of at least 12 months duration. A total of 868 patients (708 active and 160 control) were enrolled in these studies.
In the phase II study D2201 (RESOLVE), 151 patients were treated with ranibizumab (6 mg/ml, n=51, 10 mg/ml, n=51) or sham (n=49) by monthly intravitreal injections. The mean average change in BCVA from Month 1 to Month 12 compared to baseline was +7.8 (±7.72) letters in the pooled ranibizumab-treated patients (n=102), compared to -0.1 (±9.77) letters for sham-treated patients; and the mean change in BCVA at Month 12 from baseline was 10.3 (±9.1) letters compared to -1.4 (±14.2) letters, respectively (p<0.0001 for the treatment difference).
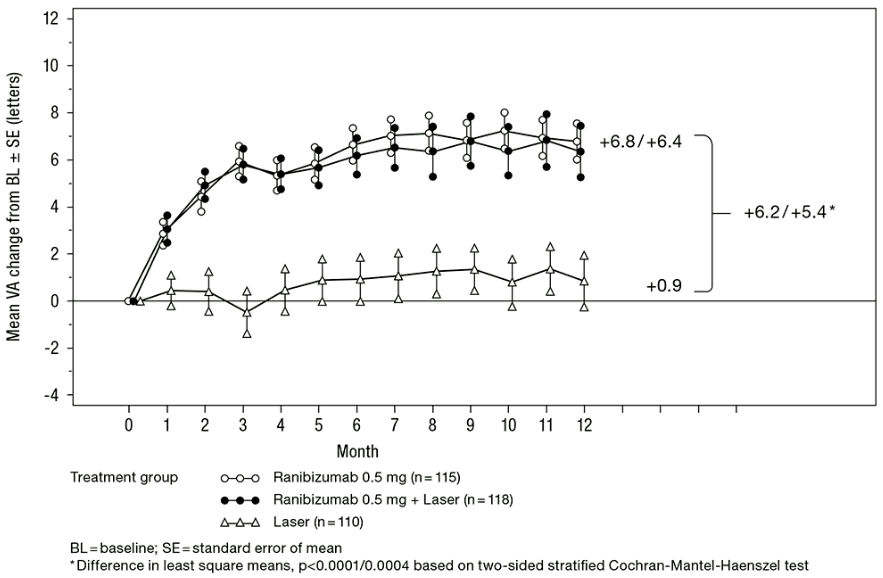
In the phase III study D2301 (RESTORE), 345 patients were randomised in a 1:1:1 ratio to receive ranibizumab 0.5 mg monotherapy and sham laser photocoagulation, combined ranibizumab 0.5 mg and laser photocoagulation or sham injection and laser photocoagulation. 240 patients, who had previously completed the 12-month RESTORE study, were enrolled in the open-label, multicentre 24-month extension (RESTORE Extension) study. Patients were treated with ranibizumab 0.5 mg pro re nata (PRN) in the same eye as the core study (D2301 RESTORE).
Key outcome measures are summarised in Table 5 (RESTORE and Extension) and Figure 4 (RESTORE).
Figure 4. Mean change in visual acuity from baseline over time in study D2301 (RESTORE):
The effect at 12 months was consistent in most subgroups. However, subjects with a baseline BCVA >73 letters and macular oedema with central retinal thickness <300 μm did not appear to benefit from treatment with ranibizumab compared to laser photocoagulation.
Table 5. Outcomes at Month 12 in study D2301 (RESTORE) and at Month 36 in study D2301-E1 (RESTORE Extension):
| Outcome measures at Month 12 compared to baseline in study D2301 (RESTORE) | Ranibizumab 0.5 mg n=115 | Ranibizumab 0.5 mg + Laser n=118 | Laser n=110 |
|---|---|---|---|
| Mean average change in BCVA from Month 1 to Month 12a (±SD) | 6.1 (6.4)a | 5.9 (7.9)a | 0.8 (8.6) |
| Mean change in BCVA at Month 12 (±SD) | 6.8 (8.3)a | 6.4 (11.8)a | 0.9 (11.4) |
| Gain of ≥15 letters or BCVA ≥84 letters at Month 12 (%) | 22.6 | 22.9 | 8.2 |
| Mean number of injections (Months 0-11) | 7.0 | 6.8 | 7.3 (sham) |
| Outcome measure at Month 36 compared to D2301 (RESTORE) baseline in study D2301-E1 (RESTORE Extension) | Prior ranibizumab 0.5 mg n=83 | Prior ranibizumab 0.5 mg + laser n=83 | Prior laser n=74 |
|---|---|---|---|
| Mean change in BCVA at Month 24 (SD) | 7.9 (9.0) | 6.7 (7.9) | 5.4 (9.0) |
| Mean change in BCVA at Month 36 (SD) | 8.0 (10.1) | 6.7 (9.6) | 6.0 (9.4) |
| Gain of ≥15 letters or BCVA ≥84 letters at Month 36 (%) | 27.7 | 30.1 | 21.6 |
| Mean number of injections (Months 12-35)* | 6.8 | 6.0 | 6.5 |
a p<0.0001 for comparisons of ranibizumab arms vs. laser arm.
n in D2301-E1 (RESTORE Extension) is the number of patients with a value at both D2301 (RESTORE) baseline (Month 0) and at the Month 36 visit.
* The proportion of patients who did not require any ranibizumab treatment during the extension phase was 19%, 25% and 20% in the prior ranibizumab, prior ranibizumab + laser and prior laser groups, respectively.
Statistically significant patient-reported benefits for most vision-related functions were observed with ranibizumab (with or without laser) treatment over the control group as measured by the NEI VFQ-25. For other subscales of this questionnaire no treatment differences could be established.
The long-term safety profile of ranibizumab observed in the 24-month extension study is consistent with the known ranibizumab safety profile.
In the phase IIIb study D2304 (RETAIN), 372 patients were randomised in 1:1:1 ratio to receive:
- ranibizumab 0.5 mg with concomitant laser photocoagulation on a treat-and-extend (TE) regimen,
- ranibizumab 0.5 mg monotherapy on a TE regimen,
- ranibizumab 0.5 mg monotherapy on a PRN regimen.
In all groups, ranibizumab was administered monthly until BCVA was stable for at least three consecutive monthly assessments. On TE, ranibizumab was administered at treatment intervals of 2-3 months. In all groups, monthly treatment was re-initiated upon a decrease in BCVA due to DME progression and continued until stable BCVA was reached again.
The number of scheduled treatment visits after the initial 3 injections, was 13 and 20 for the TE and PRN regimens, respectively. With both TE regimens, more than 70% of patients maintained their BCVA with an average visit frequency of ≥2 months.
The key outcome measures are summarised in Table 6.
Table 6. Outcomes in study D2304 (RETAIN):
| Outcome measure compared to baseline | TE ranibizumab 0.5 mg + laser n=117 | TE ranibizumab 0.5 mg alone n=125 | PRN ranibizumab 0.5 mg n=117 |
|---|---|---|---|
| Mean average change in BCVA from Month 1 to Month 12 (SD) | 5.9 (5.5)a | 6.1 (5.7)a | 6.2 (6.0) |
| Mean average change in BCVA from Month 1 to Month 24 (SD) | 6.8 (6.0) | 6.6 (7.1) | 7.0 (6.4) |
| Mean change in BCVA at Month 24 (SD) | 8.3 (8.1) | 6.5 (10.9) | 8.1 (8.5) |
| Gain of ≥15 letters or BCVA ≥84 letters at Month 24(%) | 25.6 | 28.0 | 30.8 |
| Mean number of injections (months 0-23) | 12.4 | 12.8 | 10.7 |
a p<0.0001 for assessment of non-inferiority to PRN
In DME studies, the improvement in BCVA was accompanied by a reduction over time in mean CSFT in all the treatment groups.
Treatment of PDR
The clinical safety and efficacy of ranibizumab in patients with PDR have been assessed in Protocol S which evaluated the treatment with ranibizumab 0.5 mg intravitreal injections compared with panretinal photocoagulation (PRP). The primary endpoint was the mean visual acuity change at year 2. Additionally, change in diabetic retinopathy (DR) severity was assessed based on fundus photographs using the DR severity score (DRSS).
Protocol S was a multicentre, randomised, active-controlled, parallel-assignment, non-inferiority phase III study in which 305 patients (394 study eyes) with PDR with or without DME at baseline were enrolled. The study compared ranibizumab 0.5 mg intravitreal injections to standard treatment with PRP. A total of 191 eyes (48.5%) were randomised to ranibizumab 0.5 mg and 203 eyes (51.5%) eyes were randomised to PRP. A total of 88 eyes (22.3%) had baseline DME: 42 (22.0%) and 46 (22.7%) eyes in the ranibizumab and PRP groups, respectively.
In this study, the mean visual acuity change at year 2 was +2.7 letters in the ranibizumab group compared to -0.7 letters in the PRP group. The difference in least square means was 3.5 letters (95% CI: [0.2 to 6.7]).
At year 1, 41.8% of eyes experienced a ≥2-step improvement in the DRSS when treated with ranibizumab (n=189) compared to 14.6% of eyes treated with PRP (n=199). The estimated difference between ranibizumab and laser was 27.4% (95% CI: [18.9, 35.9]).
Table 7. DRSS improvement or worsening of ≥2 or ≥3 steps at year 1 in Protocol S (LOCF Method):
| Categorised change from baseline | Protocol S | ||
|---|---|---|---|
| Ranibizumab 0.5 mg (N=189) | PRP (N=199) | Difference in proportion (%), CI | |
| ≥2-step improvement | |||
| n (%) | 79 (41.8%) | 29 (14.6%) | 27.4 (18.9, 35.9) |
| ≥3-step improvement | |||
| n (%) | 54 (28.6%) | 6 (3.0%) | 25.7 (18.9, 32.6) |
| ≥2-step worsening | |||
| n (%) | 3 (1.6%) | 23 (11.6%) | -9.9 (-14.7, -5.2) |
| ≥3-step worsening | |||
| n (%) | 1 (0.5%) | 8 (4.0%) | -3.4 (-6.3, -0.5) |
DRSS = diabetic retinopathy severity score, n = number of patients who satisfied the condition at the visit, N = total number of study eyes.
At year 1 in the ranibizumab-treated group in Protocol S, ≥2-step improvement in DRSS was consistent in eyes without DME (39.9%) and with baseline DME (48.8%).
An analysis of year 2 data from Protocol S demonstrated that 42.3% (n=80) of eyes in the ranibizumab-treated group had ≥2-step improvement in DRSS from baseline compared with 23.1% (n=46) of eyes in the PRP group. In the ranibizumab-treated group, ≥2-step improvement in DRSS from baseline was observed in 58.5% (n=24) of eyes with baseline DME and 37.8% (n=56) of eyes without DME.
DRSS was also assessed in three separate active-controlled phase III DME studies (ranibizumab 0.5 mg PRN vs laser) that included a total of 875 patients, of whom approximately 75% were of Asian origin. In a meta-analysis of these studies, 48.4% of the 315 patients with gradable DRSS scores in the subgroup of patients with moderately severe non- proliferative DR (NPDR) or worse at baseline experienced a ≥2-step improvement in the DRSS at Month 12 when treated with ranibizumab (n=192) vs 14.6% of patients treated with laser (n=123). The estimated difference between ranibizumab and laser was 29.9% (95% CI: [20.0, 39.7]). In the 405 DRSS gradable patients with moderate NPDR or better, a ≥2-step DRSS improvement was observed in 1.4% and 0.9% of the ranibizumab and laser groups, respectively.
Treatment of visual impairment due to macular oedema secondary to RVO
The clinical safety and efficacy of ranibizumab in patients with visual impairment due to macular oedema secondary to RVO have been assessed in the randomised, double-masked, controlled studies BRAVO and CRUISE that recruited subjects with BRVO (n=397) and CRVO (n=392), respectively. In both studies, subjects received either 0.3 mg or 0.5 mg ranibizumab or sham injections. After 6 months, patients in the sham-control arms switched to 0.5 mg ranibizumab.
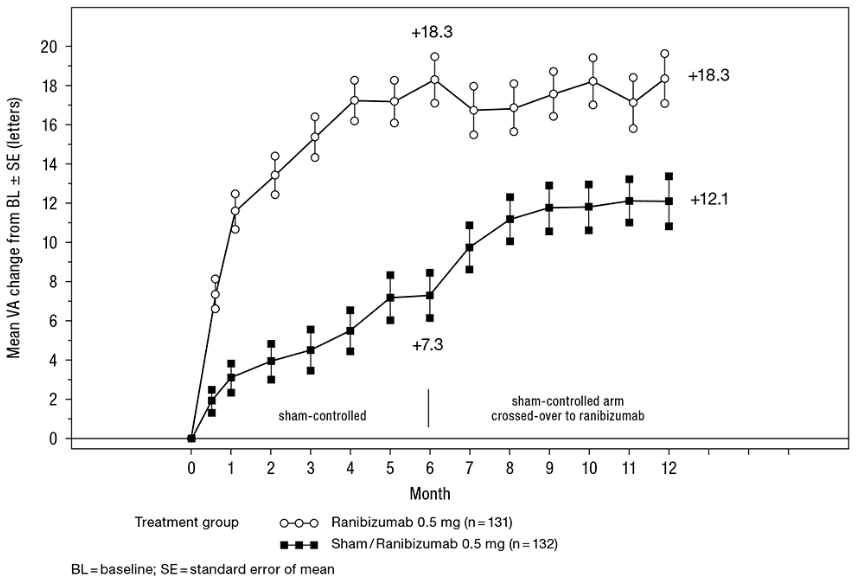
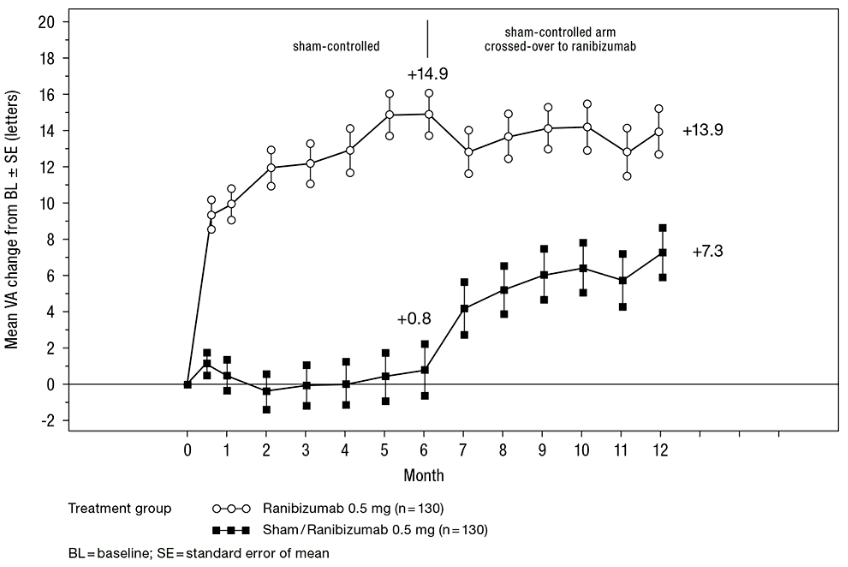
Key outcome measures from BRAVO and CRUISE are summarised in Table 8 and Figures 5 and 6.
Table 8. Outcomes at Month 6 and 12 (BRAVO and CRUISE):
| BRAVO | CRUISE | |||
|---|---|---|---|---|
| Sham/ Ranibizumab 0.5 mg (n=132) | Ranibizumab 0.5 mg (n=131) | Sham/ Ranibizumab 0.5 mg (n=130) | Ranibizumab 0.5 mg (n=130) | |
| Mean change in visual acuity at Month 6a (letters) (SD) (primary endpoint) | 7.3 (13.0) | 18.3 (13.2) | 0.8 (16.2) | 14.9 (13.2) |
| Mean change in BCVA at Month 12 (letters) (SD) | 12.1 (14.4) | 18.3 (14.6) | 7.3 (15.9) | 13.9 (14.2) |
| Gain of ≥15 letters in visual acuity at Month 6a (%) | 28.8 | 61.1 | 16.9 | 47.7 |
| Gain of ≥15 letters in visual acuity at Month 12 (%) | 43.9 | 60.3 | 33.1 | 50.8 |
| Proportion (%) receiving laser rescue over 12 months | 61.4 | 34.4 | NA | NA |
a p<0.0001 for both studies
Figure 5. Mean change from baseline BCVA over time to Month 6 and Month 12 (BRAVO):
Figure 6. Mean change from baseline BCVA over time to Month 6 and Month 12 (CRUISE):
In both studies, the improvement of vision was accompanied by a continuous and significant reduction in the macular oedema as measured by central retinal thickness.
In patients with CRVO (CRUISE and extension study HORIZON): Subjects treated with sham in the first 6 months who subsequently received ranibizumab did not achieve comparable gains in VA by Month 24 (~6 letters) compared to subjects treated with ranibizumab from study start (~12 letters).
Statistically significant patient-reported benefits in subscales related to near and distance activity were observed with ranibizumab treatment over the control group as measured by the NEI VFQ-25.
The long-term (24 months) clinical safety and efficacy of ranibizumab in patients with visual impairment due to macular oedema secondary to RVO were assessed in the BRIGHTER (BRVO) and CRYSTAL (CRVO) studies. In both studies, subjects received a 0.5 mg ranibizumab PRN dosing regimen driven by individualised stabilisation criteria. BRIGHTER was a 3-arm randomised activecontrolled study that compared 0.5 mg ranibizumab given as monotherapy or in combination with adjunctive laser photocoagulation to laser photocoagulation alone. After 6 months, subjects in the laser arm could receive 0.5 mg ranibizumab. CRYSTAL was a single-arm study with 0.5 mg ranibizumab monotherapy.
Key outcome measures from BRIGHTER and CRYSTAL are shown in Table 9.
Table 9. Outcomes at Months 6 and 24 (BRIGHTER and CRYSTAL):
| BRIGHTER | CRYSTAL | |||
|---|---|---|---|---|
| Ranibizumab 0.5 mg N=180 | Ranibizumab 0.5 mg + Laser N=178 | Laser* N=90 | Ranibizumab 0.5 mg N=356 | |
| Mean change in BCVA at Month 6a (letters) (SD) | +14.8 (10.7) | +14.8 (11.13) | +6.0 (14.27) | +12.0 (13.95) |
| Mean change in BCVA at Month 24b (letters) (SD) | +15.5 (13.91) | +17.3 (12.61) | +11.6 (16.09) | +12.1 (18.60) |
| Gain of ≥15 letters in BCVA at Month 24 (%) | 52.8 | 59.6 | 43.3 | 49.2 |
| Mean number of injections (SD) (Months 0-23) | 11.4 (5.81) | 11.3 (6.02) | NA | 13.1 (6.39) |
a p<0.0001 for both comparisons in BRIGHTER at Month 6: Ranibizumab 0.5 mg vs Laser and Ranibizumab 0.5 mg + Laser vs Laser.
b p<0.0001 for null hypothesis in CRYSTAL that the mean change at Month 24 from baseline is zero.
* Starting at Month 6 ranibizumab 0.5 mg treatment was allowed (24 patients were treated with laser only).
In BRIGHTER, ranibizumab 0.5 mg with adjunctive laser therapy demonstrated non-inferiority versus ranibizumab monotherapy from baseline to Month 24 (95% CI -2.8, 1.4).
In both studies, a rapid and statistically significant decrease from baseline in central retinal subfield thickness was observed at Month 1. This effect was maintained up to Month 24. The effect of ranibizumab treatment was similar irrespective of the presence of retinal ischaemia. In BRIGHTER, patients with ischaemia present (N=46) or absent (N=133) and treated with ranibizumab monotherapy had a mean change from baseline of +15.3 and +15.6 letters, respectively, at Month 24. In CRYSTAL, patients with ischaemia present (N=53) or absent (N=300) and treated with ranibizumab monotherapy had a mean change from baseline of +15.0 and +11.5 letters, respectively.
The effect in terms of visual improvement was observed in all patients treated with 0.5 mg ranibizumab monotherapy regardless of their disease duration in both BRIGHTER and CRYSTAL. In patients with <3 months disease duration an increase in visual acuity of 13.3 and 10.0 letters was seen at Month 1; and 17.7 and 13.2 letters at Month 24 in BRIGHTER and CRYSTAL, respectively. The corresponding visual acuity gain in patients with ≥12 months disease duration was 8.6 and 8.4 letters in the respective studies. Treatment initiation at the time of diagnosis should be considered.
The long-term safety profile of ranibizumab observed in the 24-month studies is consistent with the known ranibizumab safety profile.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with the reference medicinal product containing ranibizumab in all subsets of the paediatric population in neovascular AMD, visual impairment due to DME, visual impairment due to macular oedema secondary to RVO, visual impairment due to CNV and diabetic retinopathy (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Following monthly intravitreal administration of ranibizumab to patients with neovascular AMD, serum concentrations of ranibizumab were generally low, with maximum levels (Cmax) generally below the ranibizumab concentration necessary to inhibit the biological activity of VEGF by 50% (11-27 ng/ml, as assessed in an in vitro cellular proliferation assay). Cmax was dose proportional over the dose range of 0.05 to 1.0 mg/eye. Serum concentrations in a limited number of DME patients indicate that a slightly higher systemic exposure cannot be excluded compared to those observed in neovascular AMD patients. Serum ranibizumab concentrations in RVO patients were similar or slightly higher compared to those observed in neovascular AMD patients.
Based on analysis of population pharmacokinetics and disappearance of ranibizumab from serum for patients with neovascular AMD treated with the 0.5 mg dose, the average vitreous elimination half-life of ranibizumab is approximately 9 days. Upon monthly intravitreal administration of ranibizumab 0.5 mg/eye, serum ranibizumab Cmax, attained approximately 1 day after dosing, is predicted to generally range between 0.79 and 2.90 ng/ml, and Cmin is predicted to generally range between 0.07 and 0.49 ng/ml. Serum ranibizumab concentrations are predicted to be approximately 90,000-fold lower than vitreal ranibizumab concentrations.
Patients with renal impairment
No formal studies have been conducted to examine the pharmacokinetics of ranibizumab in patients with renal impairment. In a population pharmacokinetic analysis of neovascular AMD patients, 68% (136 of 200) of patients had renal impairment (46.5% mild [50-80 ml/min], 20% moderate [30-50 ml/min], and 1.5% severe [<30 ml/min]). In RVO patients, 48.2% (253 of 525) had renal impairment (36.4% mild, 9.5% moderate and 2.3% severe). Systemic clearance was slightly lower, but this was not clinically significant.
Hepatic impairment
No formal studies have been conducted to examine the pharmacokinetics of ranibizumab in patients with hepatic impairment.
5.3. Preclinical safety data
Bilateral intravitreal administration of ranibizumab to cynomolgus monkeys at doses between 0.25 mg/eye and 2.0 mg/eye once every 2 weeks for up to 26 weeks resulted in dose-dependent ocular effects.
Intraocularly, there were dose-dependent increases in anterior chamber flare and cells with a peak 2 days after injection. The severity of the inflammatory response generally diminished with subsequent injections or during recovery. In the posterior segment, there were vitreal cell infiltration and floaters, which also tended to be dose-dependent and generally persisted to the end of the treatment period. In the 26-week study, the severity of the vitreous inflammation increased with the number of injections. However, evidence of reversibility was observed after recovery. The nature and timing of the posterior segment inflammation is suggestive of an immune-mediated antibody response, which may be clinically irrelevant. Cataract formation was observed in some animals after a relatively long period of intense inflammation, suggesting that the lens changes were secondary to severe inflammation. A transient increase in post-dose intraocular pressure was observed following intravitreal injections, irrespective of dose.
Microscopic ocular changes were related to inflammation and did not indicate degenerative processes. Granulomatous inflammatory changes were noted in the optic disc of some eyes. These posterior segment changes diminished, and in some instances resolved, during the recovery period.
Following intravitreal administration, no signs of systemic toxicity were detected. Serum and vitreous antibodies to ranibizumab were found in a subset of treated animals.
No carcinogenicity or mutagenicity data are available.
In pregnant monkeys, intravitreal ranibizumab treatment resulting in maximal systemic exposures 0.9- 7-fold a worst case clinical exposure did not elicit developmental toxicity or teratogenicity, and had no effect on weight or structure of the placenta, although, based on its pharmacological effect ranibizumab should be regarded as potentially teratogenic and embryo-/foetotoxic.
The absence of ranibizumab-mediated effects on embryo-foetal development is plausibly related mainly to the inability of the Fab fragment to cross the placenta. Nevertheless, a case was described with high maternal ranibizumab serum levels and presence of ranibizumab in foetal serum, suggesting that the anti-ranibizumab antibody acted as (Fc region containing) carrier protein for ranibizumab, thereby decreasing its maternal serum clearance and enabling its placental transfer. As the embryofoetal development investigations were performed in healthy pregnant animals and disease (such as diabetes) may modify the permeability of the placenta towards a Fab fragment, the study should be interpreted with caution.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.