RAYALDEE Extended-release capsule Ref.[49873] Active ingredients: Calcidiol
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Calcifediol (25-hydroxyvitamin D3) is a prohormone of the active form of vitamin D3, calcitriol (1,25‑dihydroxyvitamin D3). Calcifediol is converted to calcitriol by cytochrome P450 27B1 (CYP27B1), also called 1-alpha hydroxylase, primarily in the kidney. Calcitriol binds to the vitamin D receptor in target tissues and activates vitamin D responsive pathways that result in increased intestinal absorption of calcium and phosphorus and reduced parathyroid hormone synthesis.
12.2. Pharmacodynamics
In repeat-dose clinical studies with RAYALDEE, increased levels of serum total 25-hydroxyvitamin D were associated with corresponding increases in serum total 1,25‑dihydroxyvitamin D concentrations and reductions in circulating plasma intact PTH observed within the first two weeks of RAYALDEE treatment [see Clinical Studies (14)].
12.3. Pharmacokinetics
Absorption
No food effect study was conducted with 30 mcg and 60 mcg doses of RAYALDEE. However, a food effect study with a supratherapeutic dose of 450 mcg in healthy subjects showed an approximately 5-fold increase in maximum serum calcifediol concentration (Cmax) and a 3.5-fold increase in AUC0-t when RAYALDEE was administered with a high fat, high calorie meal compared to fasting.
Exposure to calcifediol increased proportionally over the dose range of 30 to 90 mcg following repeated daily administration of RAYALDEE at bedtime to subjects with secondary hyperparathyroidism, chronic kidney disease and vitamin D insufficiency. Steady-state levels of serum total 25-hydroxyvitamin D are reached after approximately 3 months [see Clinical Studies (14)].
Distribution
Calcifediol is extensively bound to plasma proteins (>98%). The mean apparent volume of distribution is 8.8 L in healthy subjects following a single oral dose of RAYALDEE, and 30.1 L in subjects with stage 3 or 4 chronic kidney disease following repeated dosing.
Elimination
The mean elimination half-life of calcifediol is approximately 11 days in healthy individuals following a single dose of RAYALDEE, and approximately 25 days in patients with stage 3 or stage 4 chronic kidney disease following repeated once daily dosing.
Metabolism
Production of calcitriol from calcifediol is catalyzed by the 1-alpha-hydroxylase enzyme, CYP27B1, located in the kidney and other tissues. CYP24A1, located in all vitamin D-responsive tissues, catabolizes both calcifediol and calcitriol to inactive metabolites.
Excretion
Excretion of calcifediol occurs primarily through the biliary fecal route.
Specific Populations
Age, Gender and Race
Based on a population pharmacokinetic analysis, age, gender and race had no meaningful impact on steady-state concentrations of calcifediol following RAYALDEE administration.
Hepatic Impairment
The pharmacokinetics of RAYALDEE have not been investigated in patients with hepatic impairment.
Renal Impairment
Based on the population pharmacokinetics analysis, there was no meaningful difference in calcifediol steady-state concentrations following repeated RAYALDEE administration in patients with stage 3 or stage 4 chronic kidney disease.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No neoplastic changes attributable to calcifediol were observed at subcutaneous doses of 3, 10 and 33 mcg/kg/day in a 26-week rasH2 transgenic mouse study.
In vitro or in vivo mutagenicity studies have not been performed with RAYALDEE.
Calcifediol has not been shown to have significant effects on fertility in rats.
14. Clinical Studies
The efficacy and safety of RAYALDEE were evaluated in two identical multicenter, randomized, placebo-controlled, double-blind trials in patients with secondary hyperparathyroidism, stage 3 or 4 chronic kidney disease and serum total 25-hydroxyvitamin D levels between 10 and 30 ng/mL. Subjects were stratified by chronic kidney disease stage and randomized in a 2:1 ratio to receive RAYALDEE or a matching placebo at bedtime over 26 weeks. The dose of RAYALDEE was 30 mcg once daily for the first 12 weeks and either 30 or 60 mcg once daily for the last 14 weeks. The dose was increased to 60 mcg at the start of week 13 if the plasma intact PTH level was greater than 70 pg/mL, the serum 25-hydroxyvitamin D level was less than 65 ng/mL and the serum calcium level was less than 9.8 mg/dL.
A total of 213 subjects were randomized in one trial (72 received placebo and 141 received RAYALDEE), and 216 subjects were randomized in the second trial (72 received placebo and 144 received RAYALDEE). The subjects' mean age was 66 years (range 25-85), 50% were male, 65% White, 32% African-American or Black and 3% Other. At baseline, subjects had secondary hyperparathyroidism, and stage 3 (52%) or stage 4 (48%) chronic kidney disease without macroalbuminuria. The most common causes of chronic kidney disease were diabetes and hypertension and the mean estimated GFR was 31 mL/min/1.73m². Mean baseline intact PTH was 130 pg/mL for subjects with stage 3 disease (n=222) and 166 pg/mL for subjects with stage 4 disease (n=207). Mean serum calcium was 9.2 mg/dL, mean serum phosphorus was 3.7 mg/dL and mean serum 25-hydroxyvitamin D was 20 ng/mL. Of the 429 subjects randomized, 354 (83%) completed the studies.
The primary analysis compared the proportion of individuals who experienced an at least 30% reduction in plasma intact PTH from baseline to end of trial (average of weeks 20, 22, 24 and 26). A larger proportion of patients randomized to RAYALDEE experienced an at least 30% reduction in plasma intact PTH from baseline compared to placebo in both trials [33% versus 8% in the first trial (P<0.001) and 34% versus 7% in the second trial (P<0.001)].
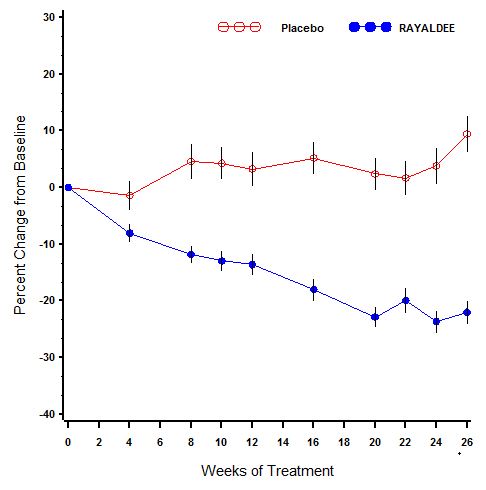
A description of mean (SE) percent change in plasma intact PTH from baseline across study visits in the two trials combined is shown in Figure 1. Serum total 25-hydroxyvitamin D levels increased to at least 30 ng/mL in 80% and 83% of subjects treated with RAYALDEE vs. 3% and 7% of subjects treated with placebo (P<0.001) in the two studies, respectively. Average steady-state 25-hydroxyvitamin D levels were 50 and 56 ng/mL for subjects receiving 30 mcg daily, and 69 and 67 ng/mL for subjects receiving 60 mcg daily, in the first and second studies, respectively.
Figure 1. Mean (±SE) Percent Change from Baseline in Plasma Intact PTH in the Per Protocol Populations (Pooled Data from Two Phase 3 Studies):
The Per Protocol (PP) population consisted of all subjects with at least 2 intact PTH values in the calculated baseline and efficacy assessment period (EAP) values and who did not have a major protocol deviation during the treatment period of the study. The PP population comprised 83% of randomized subjects.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.