REBETOL Hard capsule Ref.[8114] Active ingredients: Ribavirin
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: antivirals for systemic use, antivirals for treatment of HCV infections
ATC code: J05AP01
Mechanism of action
Ribavirin (Rebetol) is a synthetic nucleoside analogue which has shown in vitro activity against some RNA and DNA viruses. The mechanism by which Rebetol in combination with other medicinal products exerts its effects against HCV is unknown. Oral formulations of Rebetol monotherapy have been investigated as therapy for chronic hepatitis C in several clinical trials. Results of these investigations showed that Rebetol monotherapy had no effect on eliminating hepatitis virus (HCV-RNA) or improving hepatic histology after 6 to 12 months of therapy and 6 months of follow-up.
Clinical efficacy and safety
Rebetol in combination with Direct Antiviral Agent (DAA): Please refer to the SmPC of the corresponding DAA for a full description of the clinical data with such combination. Only the description of the use of Rebetol from the original development with (peg)interferon alfa-2b is detailed in the current SmPC:
Bitherapy with peginterferon alfa-2b or interferon alfa-2b: The use of Rebetol in combination treatment with peginterferon alfa-2b or interferon alfa-2b was evaluated in a number of clinical trials. Eligible patients for these trials had chronic hepatitis C confirmed by a positive HCV-RNA polymerase chain reaction assay (PCR) (>30 IU/mL), a liver biopsy consistent with a histological diagnosis of chronic hepatitis with no other cause for the chronic hepatitis, and abnormal serum ALT.
Naïve patients
Three trials examined the use of interferon in naïve patients, two with Rebetol + interferon alfa-2b (C95-132 and I95-143) and one with Rebetol + peginterferon alfa-2b (C/I98-580). In all cases the treatment was for one year with a follow-up of six months. The sustained response at the end of follow-up was significantly increased by the addition of Rebetol to interferon alfa-2b (41% vs 16%, p<0.001).
In clinical trials C95-132 and I95-143, Rebetol + interferon alfa-2b combination therapy proved to be significantly more effective than interferon alfa-2b monotherapy (a doubling in sustained response). Combination therapy also decreased the relapse rate. This was true for all HCV genotypes, particularly Genotype 1, in which the relapse rate was reduced by 30% compared with interferon alfa-2b monotherapy.
In clinical trial C/I98-580, 1,530 naïve patients were treated for one year with one of the following combination regimens:
- Rebetol (800 mg/day) + peginterferon alfa-2b (1.5 micrograms/kg/week) (n=511).
- Rebetol (1,000/1,200 mg/day) + peginterferon alfa-2b (1.5 micrograms/kg/week for one month followed by 0.5 microgram/kg/week for 11 months) (n=514).
- Rebetol (1,000/1,200 mg/day) + interferon alfa-2b (3 MIU three times a week) (n=505).
In this trial, the combination of Rebetol and peginterferon alfa-2b (1.5 micrograms/kg/week) was significantly more effective than the combination of Rebetol and interferon alfa-2b, particularly in patients infected with Genotype 1. Sustained response was assessed by the response rate six months after the cessation of treatment.
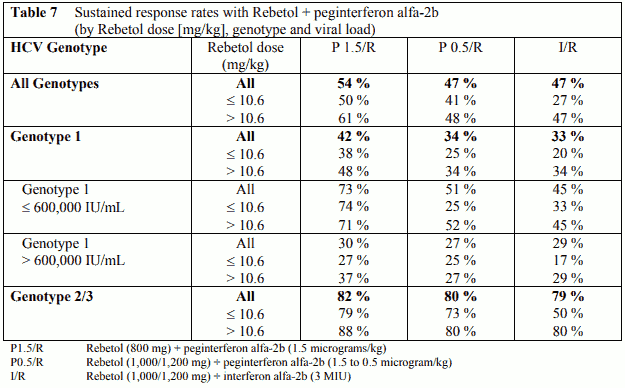
HCV genotype and baseline virus load are prognostic factors which are known to affect response rates. However, response rates in this trial were shown to be dependent also on the dose of Rebetol administered in combination with peginterferon alfa-2b or interferon alfa-2b. In those patients that received > 10.6 mg/kg Rebetol (800 mg dose in typical 75 kg patient), regardless of genotype or viral load, response rates were significantly higher than in those patients that received 10.6 mg/kg Rebetol (Table 7), while response rates in patients that received >13.2 mg/kg Rebetol were even higher.
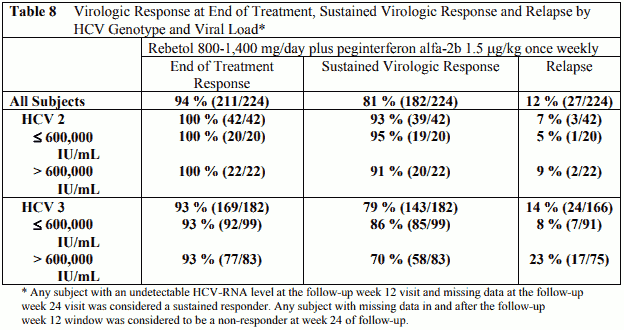
In a separate trial, 224 patients with genotype 2 or 3 received peginterferon alfa-2b, 1.5 microgram/kg subcutaneously, once weekly, in combination with ribavirin 800 mg –1,400 mg p.o. for 6 months (based on body weight, only three patients weighing >105 kg, received the 1,400 mg dose) (Table 8). Twenty-four % had bridging fibrosis or cirrhosis (Knodell ¾).
The 6 month treatment duration in this trial was better tolerated than one year of treatment in the pivotal combination trial; for discontinuation 5% vs. 14%, for dose modification 18% vs. 49%.
In a non-comparative trial, 235 patients with genotype 1 and low viral load (<600,000 IU/mL) received peginterferon alfa-2b, 1.5 microgram/kg subcutaneously, once weekly, in combination with weight adjusted Rebetol. The overall sustained response rate after a 24-week treatment duration was 50%. Forty-one percent of subjects (97/235) had nondetectable plasma HCV-RNA levels at week 4 and week 24 of therapy. In this subgroup, there was a 92% (89/97) sustained virological response rate. The high sustained response rate in this subgroup of patients was identified in an interim analysis (n=49) and prospectively confirmed (n=48). Limited historical data indicate that treatment for 48 weeks might be associated with a higher sustained response rate (11/11) and with a lower risk of relapse (0/11 as compared to 7/96 following 24 weeks of treatment).
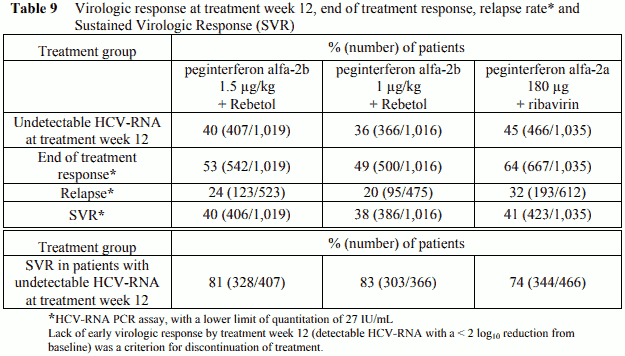
A large randomized trial compared the safety and efficacy of treatment for 48 weeks with two peginterferon alfa-2b/Rebetol regimens [peginterferon alfa-2b 1.5 µg/kg and 1 µg/kg subcutaneously once weekly both in combination with Rebetol 800 to 1,400 mg p.o. daily (in two divided doses)] and peginterferon alfa-2a 180 µg subcutaneously once weekly with ribavirin 1,000 to 1,200 mg p.o. daily (in two divided doses) in 3,070 treatment-naïve adults with chronic hepatitis C genotype 1. Response to the treatment was measured by Sustained Virologic Response (SVR) which is defined as undetectable HCV-RNA at 24 weeks post-treatment (see Table 9).
In all three treatment groups, sustained virologic response rates were similar. In patients of African American origin (which is known to be a poor prognostic factor for HCV eradication), treatment with peginterferon alfa-2b (1.5 µg/kg)/Rebetol combination therapy resulted in a higher sustained virologic response rate compared to peginterferon alfa-2b 1 µg/kg dose. At the peginterferon alfa-2b 1.5 µg/kg plus Rebetol dose, sustained virologic response rates were lower in patients with cirrhosis, in patients with normal ALT levels, in patients with a baseline viral load >600,000 IU/mL and in patients > 40 years old. Caucasian patients had a higher sustained virologic response rate compared to the African Americans. Among patients with undetectable HCV-RNA at the end of treatment, the relapse rate was 24%.
Predictability of sustained virological response in naïve patients
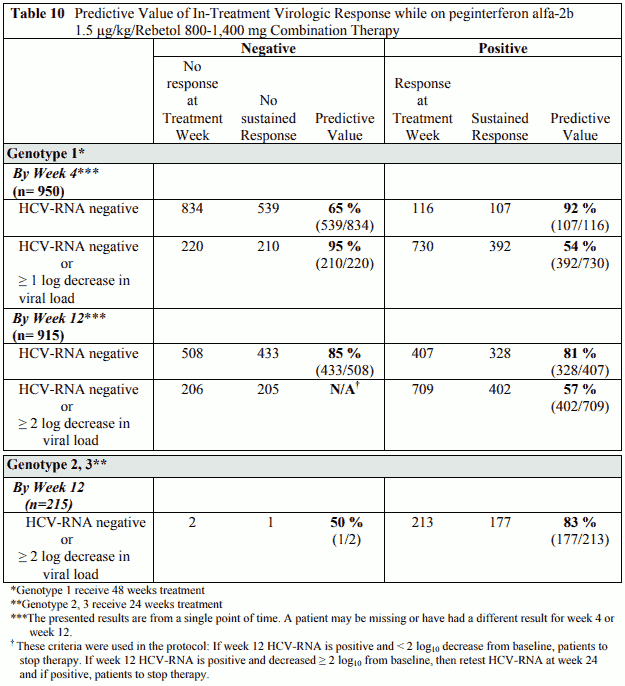
Virological response by week 12 is defined as at least 2-log viral load decrease or undetectable levels of HCV-RNA. Virological response by week 4 is defined as at least 1-log viral load decrease or undetectable levels of HCV-RNA. These time points (treatment week 4 and treatment week 12) have been shown to be predictive for sustained response (Table 10).
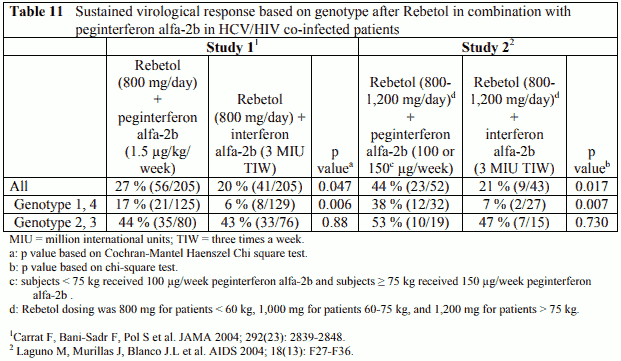
HCV/HIV Co-infected patients
Two trials have been conducted in patients co-infected with HIV and HCV. The response to treatment in both of these trials is presented in Table 11. Study 1 (RIBAVIC; P01017) was a randomized, multicentre study which enrolled 412 previously untreated adult patients with chronic hepatitis C who were co-infected with HIV. Patients were randomized to receive either Rebetol (800 mg/day) plus peginterferon alfa-2b (1.5 µg/kg/week) or Rebetol (800 mg/day) plus interferon alfa-2b (3 MIU TIW) for 48 weeks with a follow-up period of 6 months. Study 2 (P02080) was a randomized, single centre study that enrolled 95 previously untreated adult patients with chronic hepatitis C who were co-infected with HIV. Patients were randomized to receive either Rebetol (800-1,200 mg/day based on weight) plus peginterferon alfa-2b (100 or 150 µg/week based on weight) or Rebetol (800-1,200 mg/day based on weight) plus interferon alfa-2b (3 MIU TIW). The duration of therapy was 48 weeks with a follow-up period of 6 months except for patients infected with genotypes 2 or 3 and viral load <800,000 IU/mL (Amplicor) who were treated for 24 weeks with a 6 month follow-up period.
Histological response
Liver biopsies were obtained before and after treatment in Study 1 and were available for 210 of the 412 subjects (51%). Both the Metavir score and Ishak grade decreased among subjects treated with Rebetol in combination with peginterferon alfa-2b. This decline was significant among responders (-0.3 for Metavir and -1.2 for Ishak) and stable (-0.1 for Metavir and -0.2 for Ishak) among non-responders. In terms of activity, about one-third of sustained responders showed improvement and none showed worsening. There was no improvement in terms of fibrosis observed in this study. Steatosis was significantly improved in patients infected with HCV Genotype 3.
Previously treated patients
Retreatment of prior treatment failures (relapse and non-responder patients) with peginterferon alfa-2b in combination with Rebetol
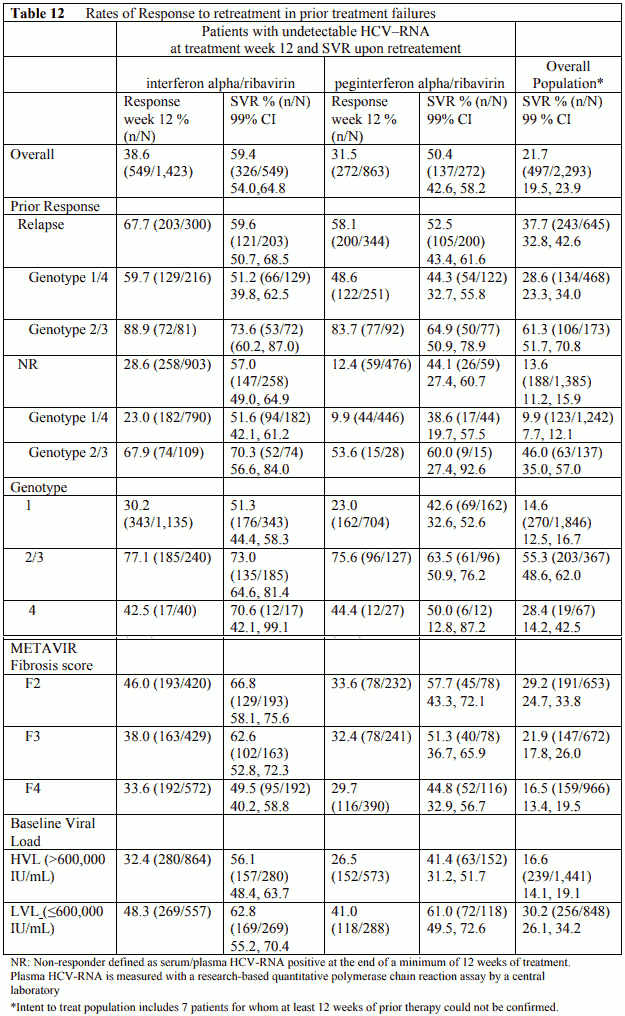
In a non-comparative trial, 2,293 patients with moderate to severe fibrosis who failed previous treatment with combination alpha interferon/ribavirin were retreated with peginterferon alfa-2b, 1.5 microgram/kg subcutaneously, once weekly, in combination with weight adjusted Rebetol. Failure to prior therapy was defined as relapse or non-response (HCV-RNA positive at the end of a minimum of 12 weeks of treatment).
Patients who were HCV-RNA negative at Treatment week 12 continued treatment for 48 weeks and were followed for 24 weeks post-treatment. Response week 12 was defined as undetectable HCV-RNA after 12 weeks of treatment. Sustained Virologic Response (SVR) is defined as undetectable HCV-RNA at 24 weeks post-treatment (Table 12).
Overall, approximately 36% (821/2,286) of patients had undetectable plasma HCV-RNA levels at week 12 of therapy measured using a research-based test (limit of detection 125 IU/mL). In this subgroup, there was a 56% (463/823) sustained virological response rate. For patients with prior failure on therapy with non-pegylated interferon or pegylated interferon and negative at week 12, the sustained response rates were 59% and 50%, respectively. Among 480 patients with > 2 log viral reduction but detectable virus at week 12, altogether 188 patients continued therapy. In those patients the SVR was 12%.
Non-responders to prior therapy with pegylated interferon alpha/ribavirin were less likely to achieve a week 12 response to retreatment than non-responders to non-pegylated interferon alpha/ribavirin (12.4% vs. 28.6%). However, if a week 12 response was achieved, there was little difference in SVR regardless of prior treatment or prior response.
Retreatment of relapse patients with Rebetol and interferon alfa-2b combination treatment
Two trials examined the use of Rebetol and interferon alfa-2b combination treatment in relapse patients (C95-144 and I95-145); 345 chronic hepatitis patients who had relapsed after previous interferon treatment were treated for six months with a six month follow-up. Combination therapy with Rebetol and interferon alfa-2b resulted in a sustained virological response that was ten-fold higher than that with interferon alfa-2b alone (49% vs 5%, p < 0.0001). This benefit was maintained irrespective of standard predictors of response to interferon alfa-2b such as virus level, HCV genotype and histological staging.
Long-term efficacy data – Adults
Two large long-term follow-up studies enrolled 1,071 patients and 567 patients after treatment in prior studies with non-pegylated interferon alfa-2b (with or without Rebetol) and pegylated interferon alfa-2b (with or without Rebetol), respectively. The purpose of the studies was to evaluate the durability of sustained virologic response (SVR) and assess the impact of continued viral negativity on clinical outcomes. At least 5 years of long-term follow-up was completed after treatment in 462 patients and 327 patients, respectively. Twelve out of 492 sustained responders and only 3 out of 366 sustained responders relapsed, respectively, in the studies.
The Kaplan-Meier estimate for continued sustained response over 5 years is 97% (95% CI: 95-99%) for patients receiving non-pegylated interferon alfa-2b (with or without Rebetol), and is 99% (95% CI: 98-100%) for patients receiving pegylated interferon alfa-2b (with or without Rebetol). SVR after treatment of chronic HCV with interferon alfa-2b (pegylated and non-pegylated, with or without Rebetol) results in long-term clearance of the virus providing resolution of the hepatic infection and clinical ‘cure’ from chronic HCV. However, this does not preclude the occurrence of hepatic events in patients with cirrhosis (including hepatocarcinoma).
Paediatric population
Clinical efficacy and safety
Rebetol in combination with peginterferon alfa-2b
Children and adolescents 3 to 17 years of age with compensated chronic hepatitis C and detectable HCV-RNA were enrolled in a multicentre trial and treated with Rebetol 15 mg/kg per day plus pegylated interferon alfa-2b 60 µg/m² once weekly for 24 or 48 weeks, based on HCV genotype and baseline viral load. All patients were to be followed for 24 weeks post-treatment. A total of 107 patients received treatment of whom 52% were female, 89% Caucasian, 67% with HCV Genotype 1 and 63% <12 years of age. The population enrolled mainly consisted of children with mild to moderate hepatitis C. Due to the lack of data in children with severe progression of the disease, and the potential for undesirable effects, the benefit/risk of the combination of Rebetol and pegylated interferon alfa-2b needs to be carefully considered in this population (see sections 4.1, 4.4 and 4.8). The study results are summarized in Table 13.
Table 13. Sustained virological response rates (na,b (%)) in previously untreated children and adolescents by genotype and treatment duration – All subjects n=107:
| 24 weeks | 48 weeks | |
|---|---|---|
| All Genotypes | 26/27 (96%) | 44/80 (55%) |
| Genotype 1 | - | 38/72 (53%) |
| Genotype 2 | 14/15 (93%) | - |
| Genotype 3c | 12/12 (100%) | ⅔ (67%) |
| Genotype 4 | - | 4/5 (80%) |
a Response to treatment was defined as undetectable HCV-RNA at 24 weeks post-treatment, lower limit of detection = 125 IU/mL.
b n = number of responders/number of subjects with given genotype, and assigned treatment duration.
c Patients with genotype 3 low viral load (<600,000 IU/mL) were to receive 24 weeks of treatment while those with genotype 3 and high viral load (≥600,000 IU/mL) were to receive 48 weeks of treatment.
Rebetol in combination with interferon alfa-2b
Children and adolescents 3 to 16 years of age with compensated chronic hepatitis C and detectable HCV-RNA (assessed by a central laboratory using a research-based RT-PCR assay) were enrolled in two multicentre trials and received Rebetol 15 mg/kg per day plus interferon alfa-2b 3 MIU/m² 3 times a week for 1 year followed by 6 months follow-up after treatment. A total of 118 patients were enrolled: 57% male, 80% Caucasian, and 78% genotype 1, 64% 12 years of age. The population enrolled mainly consisted in children with mild to moderate hepatitis C. In the two multicentre trials, sustained virological response rates in children and adolescents were similar to those in adults. Due to the lack of data in these two multicentre trials for children with severe progression of the disease, and the potential for undesirable effects, the benefit/risk of the combination of Rebetol and interferon alfa-2b needs to be carefully considered in this population (see sections 4.1, 4.4 and 4.8). The study results are summarized in Table 14.
Table 14. Sustained virological response in previously untreated children and adolescents:
| Rebetol 15 mg/kg/day + interferon alfa-2b 3 MIU/m² 3 times a week | |
|---|---|
| Overall Responsea (n=118) | 54 (46%)* |
| Genotype 1 (n=92) | 33 (36%)* |
| Genotype 2/3/4 (n=26) | 21 (81%)* |
* Number (%) of patients
a Defined as HCV-RNA below limit of detection using a research based RT-PCR assay at end of treatment and during follow-up period.
Long-term efficacy data
Rebetol in combination with peginterferon alfa-2b
A five-year long-term, observational, follow-up study enrolled 94 paediatric chronic hepatitis C patients after treatment in a multicentre trial. Of these, sixty-three were sustained responders. The purpose of the study was to annually evaluate the durability of sustained virologic response (SVR) and assess the impact of continued viral negativity on clinical outcomes for patients who were sustained responders 24 weeks post-treatment with 24 or 48 weeks of peginterferon alfa-2b and ribavirin treatment. At the end of 5 years, 85% (80/94) of all enrolled subjects and 86% (54/63) of sustained responders completed the study. No paediatric subjects with SVR relapsed during the 5 years of follow-up.
Rebetol in combination with interferon alfa-2b
A five-year long-term, observational, follow-up study enrolled 97 paediatric chronic hepatitis C patients after treatment in two previously mentioned multicentre trials. Seventy percent (68/97) of all enrolled subjects completed this study of which 75% (42/56) were sustained responders. The purpose of the study was to annually evaluate the durability of sustained virologic response (SVR) and assess the impact of continued viral negativity on clinical outcomes for patients who were sustained responders 24 weeks post-treatment of the 48-week interferon alfa-2b and ribavirin treatment. All but one of the paediatric subjects remained sustained virologic responders during long-term follow-up after completion of treatment with interferon alfa-2b plus ribavirin. The Kaplan-Meier estimate for continued sustained response over 5 years is 98% [95% CI: 95%, 100%] for paediatric patients treated with interferon alfa-2b and ribavirin. Additionally, 98% (51/52) with normal ALT levels at follow-up week 24 maintained normal ALT levels at their last visit.
SVR after treatment of chronic HCV with non-pegylated interferon alfa-2b with Rebetol results in long-term clearance of the virus providing resolution of the hepatic infection and clinical ‘cure’ from chronic HCV. However, this does not preclude the occurrence of hepatic events in patients with cirrhosis (including hepatocarcinoma).
Pharmacokinetic properties
In a single dose, crossover study of ribavirin in healthy adult subjects, the capsule and oral solution formulations were found to be bioequivalent.
Absorption
Ribavirin is absorbed rapidly following oral administration of a single dose (mean Tmax=1.5 hours), followed by rapid distribution and prolonged elimination phases (single dose half-lives of absorption, distribution and elimination are 0.05, 3.73 and 79 hours, respectively). Absorption is extensive with approximately 10% of a radiolabelled dose excreted in the faeces. However, absolute bioavailability is approximately 45%-65%, which appears to be due to first pass metabolism. There is a linear relationship between dose and AUCtf following single doses of 200-1,200 mg ribavirin. Volume of distribution is approximately 5,000 l. Ribavirin does not bind to plasma proteins.
Distribution
Ribavirin transport in non-plasma compartments has been most extensively studied in red cells, and has been identified to be primarily via an es-type equilibrative nucleoside transporter. This type of transporter is present on virtually all cell types and may account for the high volume of distribution of ribavirin. The ratio of whole blood:plasma ribavirin concentrations is approximately 60:1; the excess of ribavirin in whole blood exists as ribavirin nucleotides sequestered in erythrocytes.
Biotransformation
Ribavirin has two pathways of metabolism: 1) a reversible phosphorylation pathway; 2) a degradative pathway involving deribosylation and amide hydrolysis to yield a triazole carboxyacid metabolite. Both ribavirin and its triazole carboxamide and triazole carboxylic acid metabolites are also excreted renally.
Ribavirin has been shown to produce high inter- and intra-subject pharmacokinetic variability following single oral doses (intrasubject variability of approximately 30% for both AUC and Cmax), which may be due to extensive first pass metabolism and transfer within and beyond the blood compartment.
Elimination
Upon multiple dosing, ribavirin accumulates extensively in plasma with a six-fold ratio of multiple-dose to single-dose AUC12hr. Following oral dosing with 600 mg BID, steady-state was reached by approximately four weeks, with mean steady state plasma concentrations approximately 2,200 ng/mL. Upon discontinuation of dosing the half-life was approximately 298 hours, which probably reflects slow elimination from non-plasma compartments.
Transfer into seminal fluid
Seminal transfer of ribavirin has been studied. Ribavirin concentration in seminal fluid is approximately two-fold higher compared to serum. However, ribavirin systemic exposure of a female partner after sexual intercourse with a treated patient has been estimated and remains extremely limited compared to therapeutic plasma concentration of ribavirin.
Food effect
The bioavailability of a single oral dose of ribavirin was increased by co-administration of a high fat meal (AUCtf and Cmax both increased by 70%). It is possible that the increased bioavailability in this study was due to delayed transit of ribavirin or modified pH. The clinical relevance of results from this single dose study is unknown. In the pivotal clinical efficacy trial, patients were instructed to take ribavirin with food to achieve the maximal plasma concentration of ribavirin.
Renal function
Based on published data, single-dose ribavirin pharmacokinetics was altered (increased AUCtf and Cmax) in patients with renal dysfunction compared with control subjects (creatinine clearance >90 mL/minute). The mean AUCtf was threefold greater in subjects with creatinine clearance between 10 and 30 mL/min compared with control subjects. In subjects with creatinine clearance between 30 and 50 mL/min, AUCtf was twofold greater compared with control subjects. This appears to be due to reduction of apparent clearance in these patients. Ribavirin concentrations are essentially unchanged by haemodialysis.
Hepatic function
Single-dose pharmacokinetics of ribavirin in patients with mild, moderate or severe hepatic dysfunction (Child-Pugh Classification A, B or C) is similar to those of normal controls.
Elderly patients (≥65 years of age)
Specific pharmacokinetic evaluations for elderly subjects have not been performed. However, in a population pharmacokinetic study, age was not a key factor in the kinetics of ribavirin; renal function is the determining factor.
Population pharmacokinetic analysis was performed using sparsely sampled serum concentration values from four controlled clinical trials. The clearance model developed showed that body weight, gender, age, and serum creatinine were the main covariates. For males, clearance was approximately 20% higher than for females. Clearance increased as a function of body weight and was reduced at ages greater than 40 years. Effects of these covariates on ribavirin clearance appear to be of limited clinical significance due to the substantial residual variability not accounted for by the model.
Paediatric population
Rebetol in combination with peginterferon alfa-2b
Multiple-dose pharmacokinetic properties for Rebetol and peginterferon alfa-2b in children and adolescent patients with chronic hepatitis C have been evaluated during a clinical study. In children and adolescent patients receiving body surface area-adjusted dosing of peginterferon alfa-2b at 60 µg/m²/week, the log transformed ratio estimate of exposure during the dosing interval is predicted to be 58% (90% CI: 141-177%) higher than observed in adults receiving 1.5 µg/kg/week. The pharmacokinetics of Rebetol (dose-normalized) in this trial was similar to those reported in a prior study of Rebetol in combination with interferon alfa-2b in children and adolescent patients and in adult patients.
Rebetol in combination with interferon alfa-2b
Multiple-dose pharmacokinetic properties for Rebetol capsules and interferon alfa-2b in children and adolescents with chronic hepatitis C between 5 and 16 years of age are summarized in Table 15. The pharmacokinetics of Rebetol and interferon alfa-2b (dose-normalized) is similar in adults and children or adolescents.
Table 15. Mean (% CV) multiple-dose pharmacokinetic parameters for interferon alfa-2b and Rebetol capsules when administered to paediatric patients with chronic hepatitis C:
| Parameter | Rebetol 15 mg/kg/day as 2 divided doses (n=17) | Interferon alfa-2b 3 MIU/m² 3 times a week (n=54) |
|---|---|---|
| Tmax (hr) | 1.9 (83) | 5.9 (36) |
| Cmax (ng/mL) | 3,275 (25) | 51 (48) |
| AUC* | 29,774 (26) | 622 (48) |
| Apparent clearance L/hr/kg | 0.27 (27) | Not done |
* AUC12 (ng.hr/mL) for Rebetol; AUC0-24 (IU.hr/mL) for interferon alfa-2b
Preclinical safety data
Ribavirin
Ribavirin is embryotoxic or teratogenic, or both, at doses well below the recommended human dose in all animal species in which studies have been conducted. Malformations of the skull, palate, eye, jaw, limbs, skeleton and gastrointestinal tract were noted. The incidence and severity of teratogenic effects increased with escalation of the dose. Survival of foetuses and offspring was reduced.
In a juvenile rat toxicity study, pups dosed from postnatal day 7 to 63 with 10, 25 and 50 mg/kg of ribavirin demonstrated a dose-related decrease in overall growth, which was subsequently manifested as slight decreases in body weight, crown-rump length and bone length. At the end of the recovery period, tibial and femoral changes were minimal although generally statistically significant compared to controls in males at all dose levels and in females dosed with the two highest doses compared to controls. No histopathological effects on bone were observed. No ribavirin effects were observed regarding neurobehavioural or reproductive development. Plasma concentrations achieved in rat pups were below human plasma concentrations at the therapeutic dose.
Erythrocytes are a primary target of toxicity for ribavirin in animal studies. Anaemia occurs shortly after initiation of dosing, but is rapidly reversible upon cessation of treatment.
In 3- and 6-month studies in mice to investigate ribavirin-induced testicular and sperm effects, abnormalities in sperm, occurred at doses of 15 mg/kg and above. These doses in animals produce systemic exposures well below those achieved in humans at therapeutic doses. Upon cessation of treatment, essentially total recovery from ribavirin-induced testicular toxicity occurred within one or two spermatogenic cycles (see section 4.6).
Genotoxicity studies have demonstrated that ribavirin does exert some genotoxic activity. Ribavirin was active in the Balb/3T3 in vitro transformation assay. Genotoxic activity was observed in the mouse lymphoma assay, and at doses of 20-200 mg/kg in a mouse micronucleus assay. A dominant lethal assay in rats was negative, indicating that if mutations occurred in rats they were not transmitted through male gametes.
Conventional carcinogenicity rodent studies with low exposures compared to human exposure under therapeutic conditions (factor 0.1 in rats and 1 in mice) did not reveal tumorigenicity of ribavirin. In addition, in a 26 week carcinogenicity study using the heterozygous p53(+/-) mouse model, ribavirin did not produce tumours at the maximally tolerated dose of 300 mg/kg (plasma exposure factor approximately 2.5 compared to human exposure). These studies suggest that a carcinogenic potential of ribavirin in humans is unlikely.
Ribavirin plus interferon
When used in combination with peginterferon alfa-2b or interferon alfa-2b, ribavirin did not cause any effects not previously seen with either active substance alone. The major treatment-related change was a reversible mild to moderate anaemia, the severity of which was greater than that produced by either active substance alone.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.