REKAMBYS Prolonged-release suspension for injection Ref.[50402] Active ingredients: Rilpivirine
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
4.3. Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
REKAMBYS must not be co-administered with the following medicinal products, as significant decreases in rilpivirine plasma concentrations may occur (due to CYP3A enzyme induction), which may result in loss of therapeutic effect of REKAMBYS (see section 4.5):
- the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- the antimycobacterials rifabutin, rifampicin, rifapentine
- the systemic glucocorticoid dexamethasone, except as a single dose treatment
- St John’s wort (Hypericum perforatum).
4.4. Special warnings and precautions for use
Risk of resistance following treatment discontinuation
To minimise the risk of developing viral resistance it is essential to adopt an alternative, fully suppressive antiretroviral regimen no later than one month after the last every 1 month injection of REKAMBYS or two months after the last every 2 months injection of REKAMBYS.
If virologic failure is suspected, an alternative regimen should be adopted as soon as possible.
Long-acting properties of rilpivirine injection
Residual concentrations of rilpivirine may remain in the systemic circulation of patients for prolonged periods (up to 4 years in some patients) and should be considered upon discontinuation of REKAMBYS (see sections 4.5, 4.6, 4.7, 4.9).
Baseline factors associated with virological failure
Before starting the regimen, it should be taken into account that multivariable analyses indicate that a combination of at least 2 of the following baseline factors may be associated with an increased risk of virological failure: archived rilpivirine resistance mutations, HIV-1 subtype A6/A1, or BMI ≥30 kg/m². Available data suggest that virologic failure occurs more often when these patients are treated according to the every 2 month dosing schedule as compared to the monthly dosing regimen. In patients with an incomplete or uncertain treatment history without pre-treatment resistance analyses, caution is warranted in the presence of either BMI ≥ 30 kg/m² or HIV-1 subtype A6/A1 (see section 5.1).
Post-injection reactions
Accidental intravenous administration may result in AEs due to temporarily high plasma concentrations. In clinical studies, serious post-injection reactions were reported within minutes after the injection of rilpivirine. These events included symptoms such as dyspnoea, bronchospasm, agitation, abdominal cramping, rash/urticaria, dizziness, flushing, sweating, oral numbness, changes in blood pressure, and pain (e.g., back and chest). These events were very rare and began to resolve within minutes after the injection. Some of the patients received symptomatic treatment, at the discretion of the treating physician.
Carefully follow the Instructions for Use when preparing and administering REKAMBYS (see section 4.2). Observe patients briefly (approximately 10 minutes) after the injection. If a patient experiences a post-injection reaction, monitor and treat as clinically indicated.
Cardiovascular
REKAMBYS should be used with caution when co-administered with a medicinal product with a known risk of Torsade de Pointes. At supra-therapeutic doses (75 and 300 mg once daily), oral rilpivirine has been associated with prolongation of the QTc interval of the electrocardiogram (ECG) (see sections 4.5, 4.8 and 5.2). Oral rilpivirine at the recommended dose of 25 mg once daily is not associated with a clinically relevant effect on QTc. Plasma rilpivirine concentrations after REKAMBYS injections are comparable to those during such oral rilpivirine therapy.
HBV/HCV co-infection
Patients with hepatitis B co-infection were excluded from studies with REKAMBYS. It is not recommended to initiate REKAMBYS in patients with hepatitis B co-infection. In patients co-infected with hepatitis B receiving oral rilpivirine, the incidence of hepatic enzyme elevation was higher than in patients receiving oral rilpivirine who were not hepatitis B co-infected. Physicians should refer to current treatment guidelines for the management of HIV infection in patients co-infected with hepatitis B virus.
Limited data is available in patients with hepatitis C co-infection. In patients co-infected with hepatitis C receiving oral rilpivirine, the incidence of hepatic enzyme elevation was higher than in patients receiving oral rilpivirine who were not hepatitis C co-infected. The pharmacokinetic exposure of oral and injectable rilpivirine in co-infected patients was comparable to that in patients without hepatitis C co-infection. Monitoring of liver function is recommended in patients with hepatitis C co-infection.
Interactions with other medicinal products
REKAMBYS should not be administered with other antiretroviral medicinal products, except for cabotegravir injection for the treatment of HIV-1 infection (see section 4.5).
Pregnancy
There are limited data of REKAMBYS in pregnant women. REKAMBYS is not recommended during pregnancy unless the expected benefit justifies the potential risk. Lower exposures of oral rilpivirine were observed when rilpivirine 25 mg once daily was taken during pregnancy. In the Phase 3 studies with oral rilpivirine, lower rilpivirine exposure, similar to that seen during pregnancy, has been associated with an increased risk of virological failure, therefore viral load should be monitored closely. Alternatively, switching to another ART regimen could be considered (see sections 4.6, 5.1 and 5.2).
Immune reactivation syndrome
In HIV-infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterial infections, and Pneumocystis jirovecii pneumonia. Any inflammatory symptoms should be evaluated and treatment instituted when necessary. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution, however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Transmission of HIV
While effective viral suppression with antiretroviral therapy has been proven to substantially reduce the risk of sexual transmission, a residual risk cannot be excluded. Precautions to prevent transmission should be taken in accordance with national guidelines.
Opportunistic infections
Patients should be advised that REKAMBYS or any other antiretroviral therapy does not cure HIV infection and that they may still develop opportunistic infections and other complications of HIV infection. Therefore, patients should remain under close clinical observation by physicians experienced in the treatment of these associated HIV diseases.
Excipients
This medicine contains less than 1 mmol sodium (23 mg) per injection, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
REKAMBYS, in combination with cabotegravir injection, is intended for use as a complete regimen for the treatment of HIV-1 infection and should not be administered with other antiretroviral medicinal products for the treatment of HIV-1. Therefore, information regarding drug-drug interactions with other antiretroviral medicinal products is not provided. From a drug interaction perspective, there are no limitations on the use of other antiretroviral medicinal products after discontinuing REKAMBYS.
For the oral lead-in rilpivirine treatment and in case missed doses are replaced by oral rilpivirine treatment, refer to the oral rilpivirine tablet SmPC for information about drug interactions.
Medicinal products that affect rilpivirine exposure
Rilpivirine is primarily metabolised by cytochrome P450 (CYP)3A. Medicinal products that induce or inhibit CYP3A may thus affect the clearance of rilpivirine (see section 5.2). Co-administration of rilpivirine and medicinal products that induce CYP3A has been observed to decrease the plasma concentrations of rilpivirine, which could reduce the therapeutic effect of rilpivirine. Co-administration of rilpivirine and medicinal products that inhibit CYP3A has been observed to increase the plasma concentrations of rilpivirine.
When using oral rilpivirine, proton pump inhibitors are contraindicated (see rilpivirine tablet SmPC, section 4.3).
Medicinal products that are affected by the use of rilpivirine
Rilpivirine is not likely to have a clinically relevant effect on the exposure of medicinal products metabolised by CYP enzymes.
Rilpivirine inhibits P-glycoprotein in vitro (IC50 is 9.2 μM). In a clinical study, oral rilpivirine (25 mg once daily) did not significantly affect the pharmacokinetics of digoxin.
Rilpivirine is an in vitro inhibitor of the transporter MATE-2K with an IC50 of <2.7 nM. The clinical implications of this finding are currently unknown.
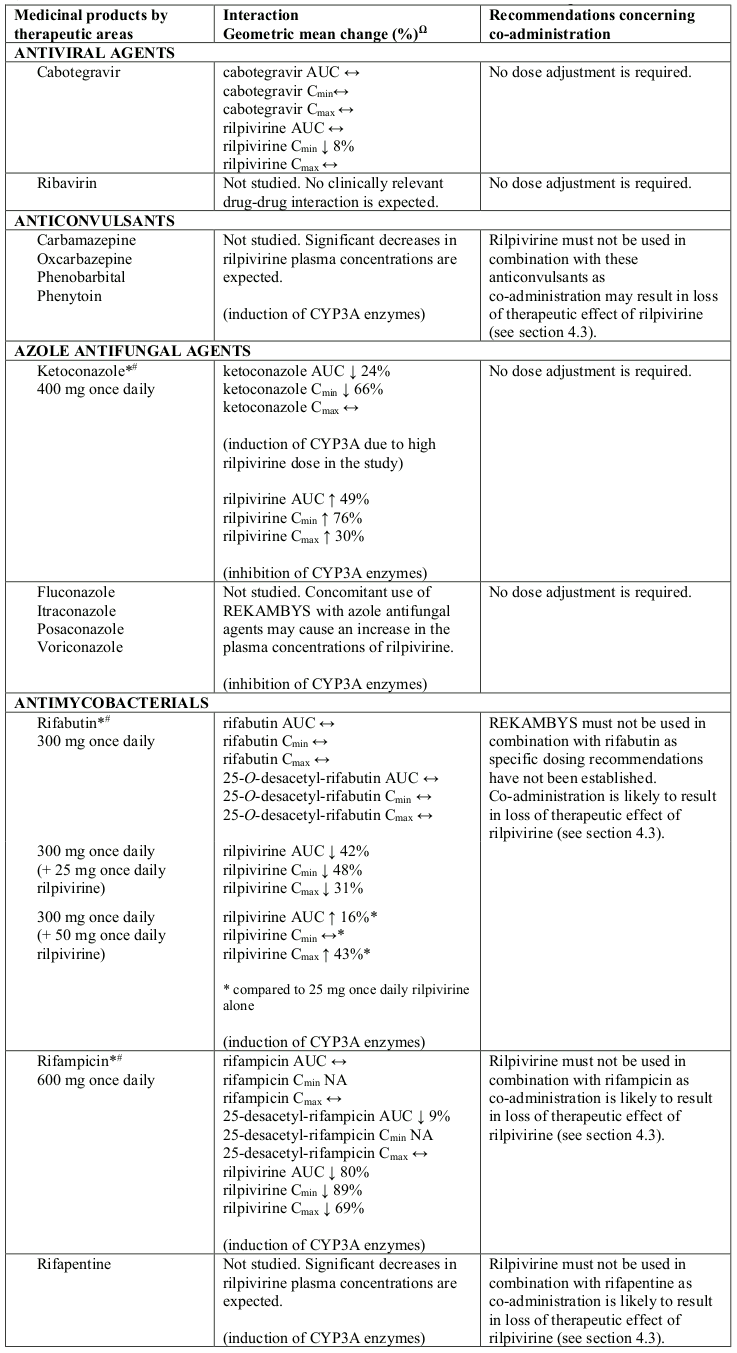
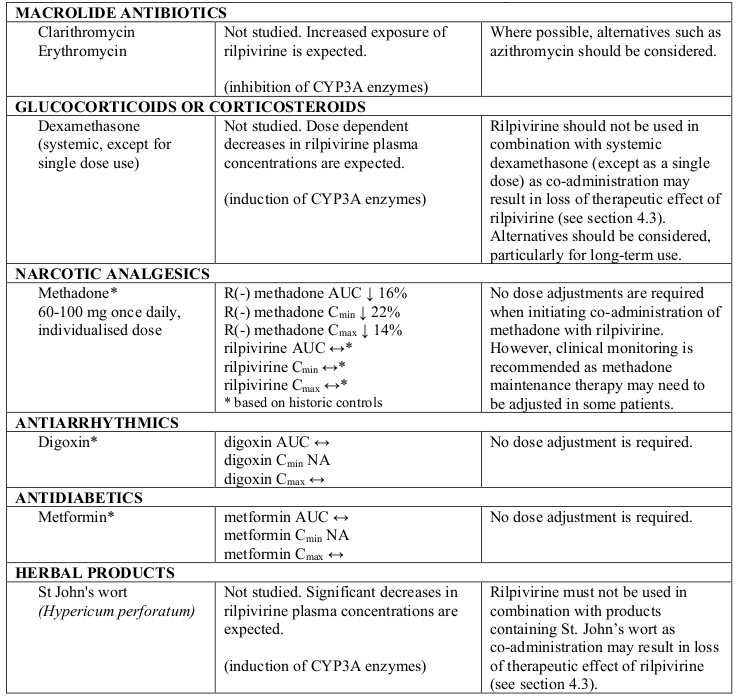
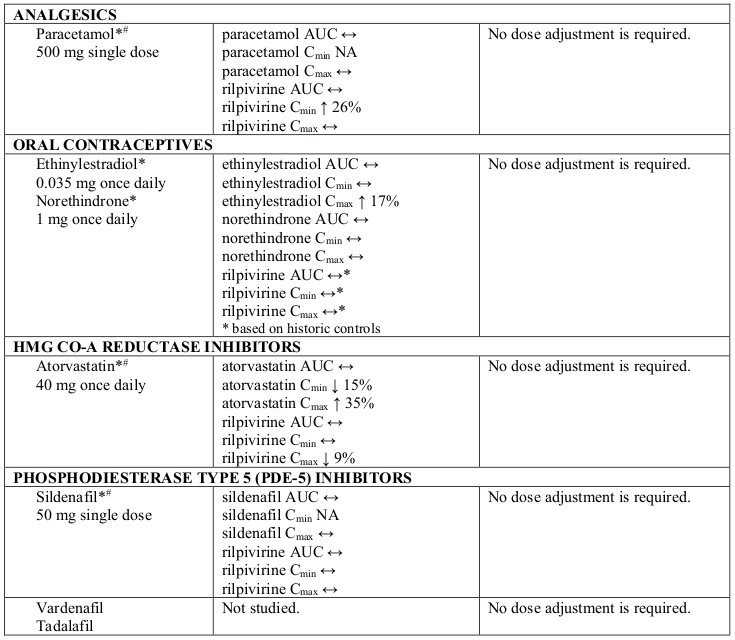
Interaction table
Selected established and theoretical interactions between rilpivirine and co-administered medicinal products are listed in Table 6 and are based on the studies conducted with oral rilpivirine or are potential drug interactions that may occur (increase is indicated as “↑”, decrease as “↓”, no change as “↔”, not applicable as “NA”, confidence interval as “CI”).
Table 6. Interactions and dose recommendations with other medicinal products:
Ω % increase/decrease based on Drug-Drug Interaction studies with oral rilpivirine
* The interaction between rilpivirine and the medicinal product was evaluated in a clinical study. All other drug-drug interactions shown are predicted.
# This interaction study has been performed with a dose higher than the recommended dose for rilpivirine assessing the maximal effect on the co-administered medicinal product. The dosing recommendation is applicable to the recommended dose of rilpivirine of 25 mg once daily.
QT prolonging medicinal products
Oral rilpivirine at the recommended dose of 25 mg once daily is not associated with a clinically relevant effect on QTc. Rilpivirine plasma concentrations after REKAMBYS injections at the recommended dose of 600 mg monthly or 900 mg every 2 months, are comparable to those achieved with oral rilpivirine at a dose of 25 mg qd. In a study of healthy subjects, supratherapeutic doses of oral rilpivirine (75 mg once daily and 300 mg once daily) have been shown to prolong the QTc interval of the ECG (see section 5.1). REKAMBYS should be used with caution when co-administered with a medicinal product with a known risk of Torsade de Pointes (see section 4.4).
4.6. Fertility, pregnancy and lactation
Pregnancy
The effect of REKAMBYS on human pregnancy is unknown.
A moderate amount of data with oral rilpivirine in pregnant women (between 300-1000 pregnancy outcomes) indicate no malformative or foetal/neonatal toxicity of rilpivirine.
A study of 19 pregnant women treated with oral rilpivirine in combination with a background regimen during the second and third trimesters, and postpartum, showed lower exposures of oral rilpivirine during pregnancy, therefore viral load should be monitored closely if REKAMBYS is used during pregnancy.
Animal studies do not indicate reproductive toxicity (see section 5.3).
REKAMBYS is not recommended during pregnancy unless the expected benefit justifies the potential risk.
An alternative oral regimen should be considered in line with current treatment guidelines. After discontinuation of REKAMBYS, rilpivirine may remain in systemic circulation for up to 4 years in some patients (see section 4.4).
Breast-feeding
It is expected that rilpivirine will be secreted into human milk based on animal data, although this has not been confirmed in humans. Rilpivirine may be present in human milk for up to 4 years in some patients after discontinuation of REKAMBYS.
It is recommended that HIV infected women do not breast-feed their infants under any circumstances in order to avoid transmission of HIV.
Fertility
No human data on the effect of rilpivirine on fertility are available. No clinically relevant effects on fertility were seen in animal studies (see section 5.3).
4.7. Effects on ability to drive and use machines
Patients should be informed that fatigue, dizziness and somnolence could occur when treated with REKAMBYS (see section 4.8).
4.8. Undesirable effects
Summary of the safety profile
The most frequently reported ARs from every 1 month dosing studies were injection site reactions (up to 84%), headache (up to 12%) and pyrexia (10%). The most frequently reported ARs from every 2 months dosing were injection site reactions (76%), headache (7%) and pyrexia (7%).
Tabulated summary of adverse reactions
The ARs identified for rilpivirine and/or cabotegravir are listed by system organ class (SOC) and frequency (see Table 7). Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10) and uncommon (≥1/1,000 to <1/100).
Table 7. Tabulated summary of adverse reactions1:
| MedDRA System Organ Class (SOC) | Frequency Category | ARs for rilpivirine + cabotegravir regimen |
|---|---|---|
| Blood and lymphatic system disorders | Common | decreased white blood cell count2, decreased haemoglobin2, decreased platelet count2 |
| Immune System Disorders | Uncommon | immune reactivation syndrome2 |
| Metabolism and nutrition disorders | Very common | increased total cholesterol (fasted)2, increased LDL cholesterol (fasted)2 |
| Common | decreased appetite2, increased triglycerides (fasted)2 | |
| Psychiatric disorders | Common | depression, anxiety, abnormal dreams, insomnia, sleep disorder2, depressed mood2 |
| Nervous system disorders | Very common | headache |
| Common | dizziness | |
| Uncommon | somnolence, vasovagal reactions (in response to injections) | |
| Gastrointestinal disorders | Very common | increased pancreatic amylase2 |
| Common | nausea, vomiting, abdominal pain3, flatulence, diarrhoea, abdominal discomfort2, dry mouth2, increased lipase2 | |
| Hepatobiliary disorders | Uncommon | hepatotoxicity |
| Skin and subcutaneous tissue disorders | Common | rash4 |
| Musculoskeletal and connective tissue disorders | Common | myalgia |
| General disorders and administrative site conditions | Very common | injection site reactions (pain and discomfort, nodule, induration), pyrexia5 |
| Common | injection site reactions (swelling, erythema, pruritus, bruising, warmth, haematoma), fatigue, asthenia, malaise | |
| Uncommon | injection site reactions (cellulitis, abscess, anaesthesia, haemorrhage, discolouration) | |
| Investigations | Common | weight increased |
| Uncommon | transaminase increased, blood bilirubin increased |
1 The frequency of the identified ARs are based on all reported occurrences of the events and are not limited to those considered at least possibly related by the investigator.
2 Additional adverse reactions seen with oral rilpivirine in other studies.
3 Abdominal pain includes the following grouped MedDRA preferred term: abdominal pain, upper abdominal pain.
4 Rash includes the following grouped MedDRA preferred terms: rash, rash erythematous, rash generalised, rash macular, rash maculo-papular, rash morbilliform, rash papular, rash pruritic.
5 Pyrexia includes the following grouped MedDRA preferred terms: pyrexia, feeling hot, body temperature increased. The majority of pyrexia events were reported within one week of injections.
The overall safety profile at Week 96 and Week 124 in the FLAIR study was consistent with that observed at Week 48, with no new safety findings identified. In the extension phase of the FLAIR study, initiating the rilpivirine plus cabotegravir injection regimen without oral lead-in (direct to injection) was not associated with any new safety concerns related to omitting the oral lead-in phase.
Description of selected adverse reactions
Local Injection Site Reactions (ISRs)
Up to 1% of subjects discontinued treatment with rilpivirine and cabotegravir injections because of ISRs.
Injection site reactions were generally mild (Grade 1, 70%-75% of subjects) or moderate (Grade 2, 27%-36% of subjects). 3-4% of subjects experienced severe (Grade 3) ISRs. The median duration of ISR events was 3 days. The percentage of subjects reporting ISRs decreased over time.
Weight increased
At the Week 48 time point, subjects in Phase 3 Studies FLAIR and ATLAS, who received rilpivirine plus cabotegravir gained a median of 1.5 kg in weight; subjects continuing on their current antiretroviral regimen (CAR) group gained a median of 1.0 kg (pooled analysis).
In the individual studies FLAIR and ATLAS, the median weight gains in the rilpivirine plus cabotegravir arms were 1.3 kg and 1.8 kg, respectively, compared to 1.5 kg and 0.3 kg in the CAR arms.
At the 48 week timepoint, in ATLAS-2M the median weight gain in both the monthly and every 2 months rilpivirine+cabotegravir dosing arms was 1.0 kg.
Changes in laboratory chemistry
Elevated transaminases (ALT/AST) were observed in subjects receiving rilpivirine plus cabotegravir during the clinical studies. These elevations were primarily attributed to acute viral hepatitis. A few subjects on oral rilpivirine plus oral cabotegravir treatment had transaminase elevations attributed to suspected drug-related hepatotoxicity; these changes were reversible upon discontinuation of treatment.
Small, non-progressive increases in total bilirubin (without clinical jaundice) were observed with treatment with rilpivirine plus cabotegravir. These changes are not considered clinically relevant as they likely reflect competition between cabotegravir and unconjugated bilirubin for a common clearance pathway (UGT1A1).
Elevated lipases were observed during clinical trials with rilpivirine plus cabotegravir. Grade 3 and 4 lipase increases occurred at a higher incidence with rilpivirine plus cabotegravir compared with CAR. These elevations were generally asymptomatic and did not lead to rilpivirine plus cabotegravir discontinuation. One case of fatal pancreatitis with Grade 4 lipase and confounding factors (including history of pancreatitis) has been reported in study ATLAS-2M for which the causality to the injection regimen could not be ruled out.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
This medicinal product must not be mixed with other medicinal products or diluents.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.