RELYVRIO Powder for oral suspension Ref.[50247] Active ingredients: Sodium phenylbutyrate Ursodoxicoltaurine
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
RELYVRIO contains two active ingredients: sodium phenylbutyrate and taurursodiol.
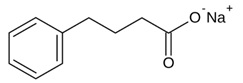
The chemical designation for phenylbutyrate is 4-phenyl butyric acid sodium salt. Its molecular formula is C10H11NaO2, and its molecular weight is 186.2.
The sodium phenylbutyrate chemical structure is:
Sodium phenylbutyrate is a white or yellow powder which decomposes at about 220°C. It is freely soluble in water and methanol; sparingly soluble in ethanol; and practically insoluble in methylene chloride, acetone, and diethyl ether.
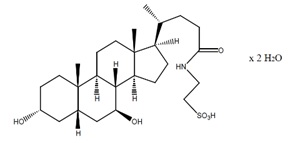
Taurursodiol, also known as tauroursdeoxycholic acid, is an ambiphilic bile acid and is the taurine conjugate of ursodiol, also known as ursodeoxycholic acid. The chemical name of taurursodiol is 2-[(3α, 7β-dihydroxy-24-oxo-5β-cholan-24-yl) amino] ethane sulfonic acid, dihydrate. The molecular formula is C26H45NO6S . 2H2O and the molecular weight is 535.74 (dihydrate).
The taurursodiol chemical structure is:
Taurursodiol is a white microcrystalline powder, practically odorless, with a bitter taste. It is freely soluble in ethyl alcohol, very slightly soluble in acetone and dioxane, sparingly soluble in water, and practically insoluble in ether and ethyl acetate.
RELYVRIO is a white to yellow powder for oral suspension that consists of fine to large granules. RELYVRIO is supplied in a packet containing 3 g sodium phenylbutyrate (equivalent to 2630 mg phenylbutyrate) and 1 g taurursodiol. Each packet contains 464 mg of sodium and also contains the following inactive ingredients: acacia, dextrates, dibasic sodium phosphate, maltodextrin, medium-chain triglycerides, mixed berry flavoring, other flavoring ingredients, silicon dioxide, sodium stearyl fumarate, sorbitol, and sucralose.
| Dosage Forms and Strengths |
|---|
|
For oral suspension: white to yellow powder provided in single-dose packets each containing 3 g sodium phenylbutyrate and 1 g taurursodiol. |
| How Supplied |
|---|
|
RELYVRIO for oral suspension is supplied in single-dose packets of white to yellow powder containing 3 g sodium phenylbutyrate and 1 g taurursodiol as follows:
Manufactured for and Distributed by: Amylyx Pharmaceuticals, Inc., 43 Thorndike Street, Cambridge, MA 02141. |
Drugs
| Drug | Countries | |
|---|---|---|
| RELYVRIO | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.