RETEVMO Capsule Ref.[10397] Active ingredients: Selpercatinib
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
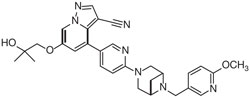
Selpercatinib is a kinase inhibitor. The molecular formula for selpercatinib is C29H31N7O3 and the molecular weight is 525.61 g/mol. The chemical name is 6-(2-hydroxy-2-methylpropoxy)-4-(6-(6-((6-methoxypyridin-3-yl)methyl)-3,6-diazabicyclo[3.1.1]heptan-3-yl)pyridin-3-yl)pyrazolo[1,5-a]pyridine-3-carbonitrile. Selpercatinib has the following chemical structure:
Selpercatinib is a white to light yellow powder that is slightly hygroscopic. The aqueous solubility of selpercatinib is pH dependent, from freely soluble at low pH to slightly soluble at neutral pH.
RETEVMO (selpercatinib) is supplied as 40 mg or 80 mg hard gelatin capsules for oral use. Each capsule contains inactive ingredients of microcrystalline cellulose and colloidal silicon dioxide. The 40 mg capsule shell is composed of gelatin, titanium dioxide, ferric oxide black and black ink. The 80 mg capsule shell is composed of gelatin, titanium dioxide, FD&C blue #1 and black ink. The black ink is composed of shellac, potassium hydroxide and ferric oxide black.
| Dosage Forms and Strengths |
|---|
|
Capsules:
|
| How Supplied |
|---|
|
RETEVMO (selpercatinib) capsules are supplied as follows: 40 mg: Gray opaque, imprinted with "Lilly", "3977" and "40 mg" in black ink. 60 count bottle - NDC# 0002-3977-60 80 mg: Blue opaque, imprinted with "Lilly", "2980" and "80 mg" in black ink. 60 count bottle - NDC# 0002-2980-60 Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| RETEVMO | Israel, Japan, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.