REXULTI Tablet Ref.[11112] Active ingredients: Brexpiprazole
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
The mechanism of action of brexpiprazole in the treatment of major depressive disorder or schizophrenia is unknown. However, the efficacy of brexpiprazole may be mediated through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors, and antagonist activity at serotonin 5-HT2A receptors.
12.2. Pharmacodynamics
Brexpiprazole has affinity (expressed as Ki) for multiple monoaminergic receptors including serotonin 5-HT1A (0.12 nM), 5-HT2A (0.47 nM), 5-HT2B (1.9 nM), 5-HT7 (3.7 nM), dopamine D2 (0.30 nM), D3 (1.1 nM), and noradrenergic α1A (3.8 nM), α1B (0.17 nM), α1D (2.6 nM), and α2C (0.59 nM) receptors. Brexpiprazole acts as a partial agonist at the 5-HT1A, D2, and D3 receptors and as an antagonist at 5-HT2A, 5-HT2B, 5-HT7, α1A, α1B, α1D, and α2C receptors. Brexpiprazole also exhibits affinity for histamine H1 receptor (19 nM) and for muscarinic M1 receptor (67% inhibition at 10 µM).
Cardiac Electrophysiology
At a dose 3 times the MRHD for the treatment of schizophrenia and 4 times the MRHD for adjunctive therapy to antidepressants for the treatment of MDD, REXULTI does not prolong the QTc interval to any clinically relevant extent.
12.3. Pharmacokinetics
Absorption
After single-dose administration of REXULTI tablets, the peak plasma brexpiprazole concentrations occurred within 4 hours after administration, and the absolute oral bioavailability was 95%. Brexpiprazole steady-state concentrations were attained within 10 to 12 days of dosing.
REXULTI can be administered with or without food. Administration of a 4 mg REXULTI tablet with a standard high-fat meal did not significantly affect the Cmax or AUC of brexpiprazole. After single and multiple once daily dose administration, brexpiprazole exposure (Cmax and AUC) increased in proportion to the dose administered. In vitro studies of brexpiprazole did not indicate that brexpiprazole is a substrate of efflux transporters such as MDRI (P-gp) and BCRP.
Distribution
The volume of distribution of brexpiprazole following intravenous administration is high (1.56±0.42 L/kg), indicating extravascular distribution. Brexpiprazole is highly protein bound in plasma (greater than 99%) to serum albumin and α1-acid glycoprotein, and its protein binding is not affected by renal or hepatic impairment. Based on results of in vitro studies, brexpiprazole protein binding is not affected by warfarin, diazepam, or digitoxin.
Elimination
Metabolism
Based on in vitro metabolism studies of brexpiprazole using recombinant human cytochrome P450 (CYP1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4), the metabolism of brexpiprazole was shown to be mainly mediated by CYP3A4 and CYP2D6.
In vivo brexpiprazole is metabolized primarily by CYP3A4 and CYP2D6 enzymes. After single- and multiple-dose administrations, brexpiprazole and its major metabolite, DM-3411, were the predominant drug moieties in the systemic circulation. At steady-state, DM-3411 represented 23% to 48% of brexpiprazole exposure (AUC) in plasma. DM-3411 is considered not to contribute to the therapeutic effects of brexpiprazole.
Based on in vitro data, brexpiprazole showed little to no inhibition of CYP450 isozymes.
Excretion
Following a single oral dose of [14C]-labeled brexpiprazole, approximately 25% and 46% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 1% of unchanged brexpiprazole was excreted in the urine, and approximately 14% of the oral dose was recovered unchanged in the feces. Apparent oral clearance of a brexpiprazole oral tablet after once daily administration is 19.8 (±11.4) mL/h/kg. After multiple once daily administration of REXULTI, the terminal elimination half-lives of brexpiprazole and its major metabolite, DM-3411, were 91 hours and 86 hours, respectively.
Studies in Specific Populations
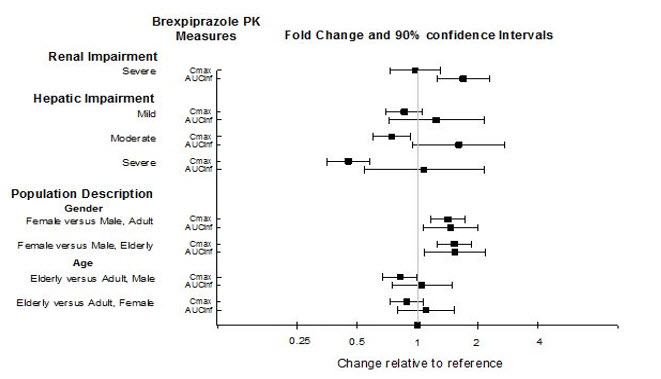
Exposures of brexpiprazole in specific populations are summarized in Figure 1. Population pharmacokinetic (PK) analysis indicated exposure of brexpiprazole in patients with moderate renal impairment was higher compared to patients with normal renal function.
Figure 1. Effects of Intrinsic Factors on Brexpiprazole Pharmacokinetics:
Drug Interaction Studies
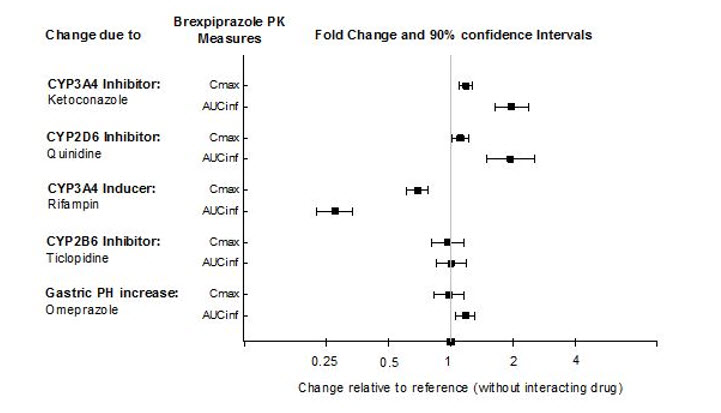
Effects of other drugs on the exposures of brexpiprazole are summarized in Figure 2. Based on simulation, a 5.1-fold increase in AUC values at steady-state is expected when extensive metabolizers of CYP2D6 are administered with both strong CYP2D6 and CYP3A4 inhibitors. A 4.8-fold increase in mean AUC values at steady-state is expected in poor metabolizers of CYP2D6 administered with strong CYP3A4 inhibitors [see Drug Interactions (7.1)].
Figure 2. The Effects of Other Drugs on Brexpiprazole Pharmacokinetics:
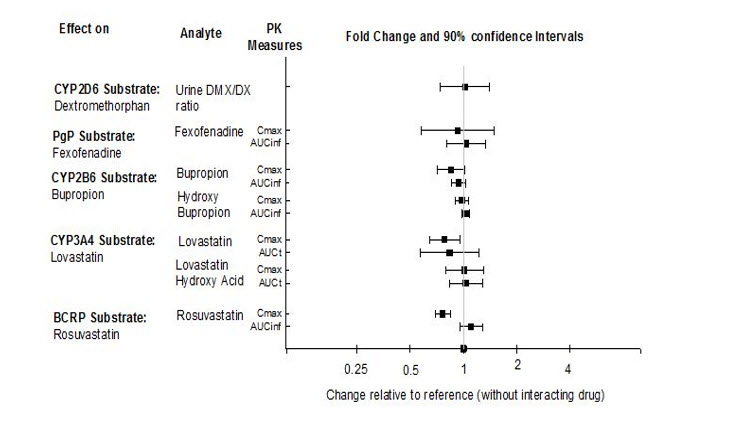
The effects of REXULTI on the exposures of other drugs are summarized in Figure 3.
Figure 3. The Effects of REXULTI on Pharmacokinetics of Other Drugs:
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted in ICR mice and Sprague Dawley rats. Brexpiprazole was administered orally for two years to male and female mice at doses of 0.75, 2, and 5 mg/kg/day (0.9 to 6.1 times the oral MRHD of 4 mg/day based on mg/m² body surface area) and to male and female rats at doses of 1, 3, and 10 mg/kg and 3, 10, and 30 mg/kg/day, respectively (2.4 to 24 and 7.3 to 73 times the oral MRHD, males and females). In female mice, the incidence of mammary gland adenocarcinoma was increased at all doses, and the incidence of adenosquamous carcinoma was increased at 2.4 and 6.1 times the MRHD. No increase in the incidence of tumors was observed in male mice. In the rat study, brexpiprazole was not carcinogenic in either sex at doses up to 73 times the MRHD.
Proliferative and/or neoplastic changes in the mammary and pituitary glands of rodents have been observed following chronic administration of antipsychotic drugs and are considered to be prolactin mediated. The potential for increasing serum prolactin level of brexpiprazole was shown in both mice and rats. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unknown.
Mutagenesis
Brexpiprazole was not mutagenic when tested in the in vitro bacterial reverse mutation assay (Ames test). Brexpiprazole was negative for clastogenic activity in the in vivo micronucleus assay in rats and was not genotoxic in the in vivo/in vitro unscheduled DNA synthesis assay in rats. In vitro with mammalian cells brexpiprazole was clastogenic but only at doses that induced cytotoxicity. Based on a weight of evidence, brexpiprazole is not considered to present a genotoxic risk to humans.
Impairment of Fertility
Female rats were treated with oral doses of 0.3, 3, or 30 mg/kg/day (0.7, 7.3, and 73 times the oral MRHD on a mg/m² basis) prior to mating with untreated males and continuing through conception and implantation. Estrus cycle irregularities and decreased fertility were observed at 3 and 30 mg/kg/day. Prolonged duration of pairing and increased preimplantation losses were observed at 30 mg/kg/day.
Male rats were treated with oral doses of 3, 10, or 100 mg/kg/day (7.3, 24, and 240 times the oral MRHD on a mg/m² basis) for 63 days prior to mating with untreated females and throughout the 14 days of mating. No differences were observed in the duration of mating or fertility indices in males at any dose of brexpiprazole.
14. Clinical Studies
14.1 Adjunctive Treatment of Major Depressive Disorder
The efficacy of REXULTI in the adjunctive treatment of major depressive disorder (MDD) was evaluated in two 6-week double-blind, placebo-controlled, fixed-dose trials of adult patients meeting DSM-IV-TR criteria for MDD, with or without symptoms of anxiety, who had an inadequate response to prior antidepressant therapy (1 to 3 courses) in the current episode and who had also demonstrated an inadequate response throughout the 8 weeks of prospective antidepressant treatment (with escitalopram, fluoxetine, paroxetine controlled-release, sertraline, duloxetine delayed release, or venlafaxine extended release). Inadequate response during the prospective antidepressant treatment phase was defined as having persistent symptoms without substantial improvement throughout the course of treatment.
Patients in Study 228 (hereafter “Study 1”) were randomized to REXULTI 2 mg once a day or placebo. Patients in Study 227 (hereafter “Study 2”) were randomized to REXULTI 1 or 3 mg once a day or placebo. For patients randomized to REXULTI, all patients initiated treatment at 0.5 mg once daily during Week 1. At Week 2, the REXULTI dosage was increased to 1 mg in all treatment groups, and either maintained at 1 mg or increased to 2 mg or 3 mg once daily, based on treatment assignment, from Week 3 onwards. The dosages were then maintained for the 4 remaining weeks.
The primary endpoint was change from baseline to Week 6 in the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-related scale used to assess the degree of depressive symptomatology, with 0 representing no symptoms and 60 representing worst symptoms.
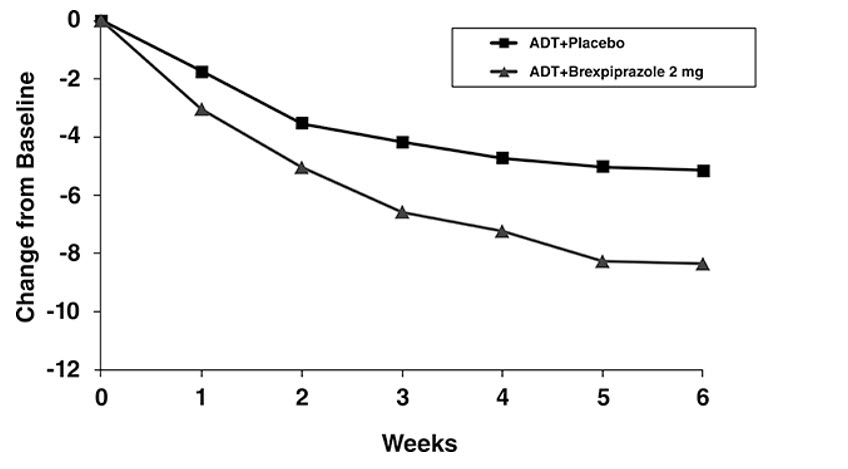
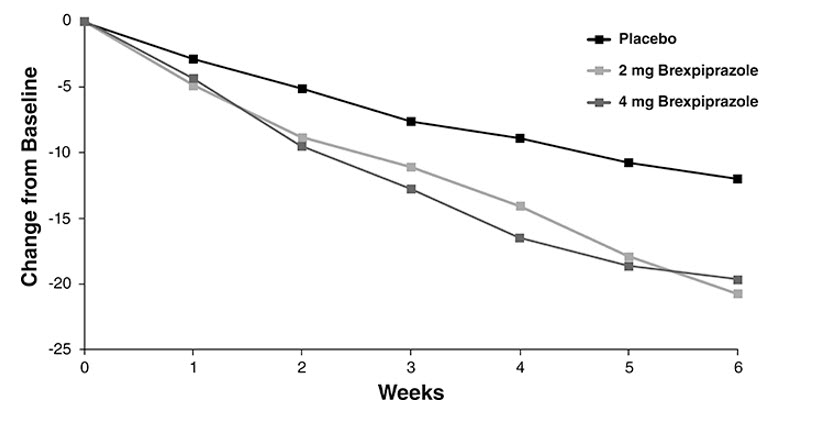
At randomization, the mean MADRS total score was 27. In Studies 1 and 2, REXULTI [+ antidepressant (ADT)] 2 mg/day and 3 mg/day were superior to placebo + ADT in reducing mean MADRS total scores. Results from the primary efficacy parameters for both fixed dose trials are shown below in Table 11. Figure 4 below shows the time course of response based on the primary efficacy measure (MADRS) in Study 1.
Table 11. Summary of Efficacy Results for Studies 1 and 2 for the Adjunctive Treatment of MDD:
| Study | Treatment Group | N | Primary Efficacy Measure: MADRS | ||
|---|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference* (95% CI) | |||
| 1 | REXULTI (2 mg/day) + ADT† | 175 | 26.9 (5.7) | -8.4 (0.6) | -3.2 (-4.9, -1.5) |
| Placebo + ADT | 178 | 27.3 (5.6) | -5.2 (0.6) | -- | |
| 2 | REXULTI (1 mg/day) + ADT | 211 | 26.5 (5.6) | -7.6 (0.5) | -1.3 (-2.7, 0.1) |
| REXULTI (3 mg/day) + ADT | 213 | 26.5 (5.3) | -8.3 (0.5) | -2.0 (-3.4, -0.5) | |
| Placebo + ADT | 203 | 26.5 (5.2) | -6.3 (0.5) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval
* Difference (drug minus placebo) in least-squares mean change from baseline
† Dosages statistically significantly superior to placebo
An examination of population subgroups did not suggest differential response based on age, gender, race or choice of prospective antidepressant.
Figure 4. Change from Baseline in MADRS Total Score by Study Visit (Week) in Patients with MDD in Study 1:
14.2 Schizophrenia
The efficacy of REXULTI in the treatment of adults with schizophrenia was demonstrated in two 6-week randomized, double-blind, placebo-controlled, fixed-dose clinical trials in patients who met DSM-IV-TR criteria for schizophrenia.
In both studies, Study 231 (hereafter “Study 3”) and Study 230 (hereafter “Study 4”), patients were randomized to REXULTI 2 or 4 mg once per day or placebo. Patients in the REXULTI groups initiated treatment at 1 mg once daily on Days 1 to 4. The REXULTI dosage was increased to 2 mg on Days 5 to 7. The dosage was then either maintained at 2 mg once daily or increased to 4 mg once daily, depending on treatment assignment, for the 5 remaining weeks.
The primary efficacy endpoint of both trials was the change from baseline to Week 6 in the Positive and Negative Syndrome Scale (PANSS) total score. The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 (best) to 210 (worst).
In Study 3, REXULTI at both 2 mg/day and 4 mg/day was superior to placebo on the PANSS total score. In Study 4, REXULTI 4 mg/day was superior to placebo on the PANSS total score (Table 12). Figure 5 shows the time course of response based on the primary efficacy measure (change from baseline in PANSS total score) in Study 3.
Examination of population subgroups based on age, gender and race did not suggest differential responsiveness.
Table 12. Summary of Efficacy Results for Studies in Schizophrenia:
| Study | Treatment Group | N | Primary Efficacy Measure: PANSS | ||
|---|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference* (95% CI) | |||
| 3 | REXULTI (2 mg/day)† | 180 | 95.9 (13.8) | -20.7 (1.5) | -8.7 (-13.1, -4.4) |
| REXULTI (4 mg/day)† | 178 | 94.7 (12.1) | -19.7 (1.5) | -7.6 (-12.0, -3.1) | |
| Placebo | 178 | 95.7 (11.5) | -12.0 (1.6) | -- | |
| 4 | REXULTI (2 mg/day) | 179 | 96.3 (12.9) | -16.6 (1.5) | -3.1 (-7.2, 1.1) |
| REXULTI (4 mg/day)† | 181 | 95.0 (12.4) | -20.0 (1.5) | -6.5 (-10.6, -2.4) | |
| Placebo | 180 | 94.6 (12.8) | -13.5 (1.5) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval
* Difference (drug minus placebo) in least-squares mean change from baseline
† Dosages statistically significantly superior to placebo
Figure 5. Change from Baseline in PANSS Total Score by Study Visit (Week) in Patients with Schizophrenia in Study 3:
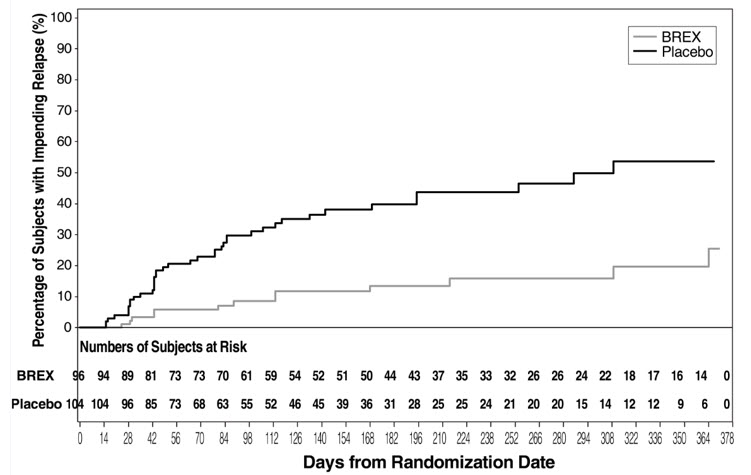
The safety and efficacy of REXULTI as maintenance treatment in adults with schizophrenia aged 18 to 65 years were demonstrated in the maintenance phase of a randomized withdrawal trial (Study 331-10-232, hereafter “Study 5”). Patients were stabilized for at least 12 weeks on 1 to 4 mg/day of REXULTI (N=202). They were then randomized in the double-blind treatment phase to either continue REXULTI at their achieved stable dose (N=97), or to switch to placebo (N=105).
The primary endpoint in Study 5 was time from randomization to impending relapse during the double-blind phase, defined as: 1) Clinical Global Improvement score of ≥5 (minimally worse) and an increase to a score >4 on PANSS conceptual disorganization, hallucinatory behavior, suspiciousness, or unusual thought content items, with either a ≥2 increase on a specific item or ≥4 point increase on the combined four PANSS items, 2) hospitalization due to worsening of psychotic symptoms, 3) current suicidal behavior, or 4) violent/aggressive behavior.
A pre-specified interim analysis demonstrated a statistically significantly longer time to relapse in patients randomized to the REXULTI group compared to placebo-treated patients. The trial was subsequently terminated early because maintenance of efficacy had been demonstrated. The Kaplan-Meier curves of the cumulative proportion of patients with relapse during the double-blind treatment phase for REXULTI and placebo groups are shown in Figure 6. The key secondary endpoint, the proportion of subjects who met the criteria for impending relapse, was statistically significantly lower in REXULTI-treated patients compared with placebo group.
Figure 6. Kaplan-Meier Estimation of Percent Impending Relapse in Study 5:
Note: A total of 202 subjects were randomized. Among them, one placebo subject did not take investigational medicinal product and one brexpiprazole subject did not have post-randomization efficacy evaluations. These two subjects were excluded from the efficacy analysis.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.