REYVOW Tablet Ref.[10110] Active ingredients: Lasmiditan
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Lasmiditan binds with high affinity to the 5-HT1F receptor. Lasmiditan presumably exerts its therapeutic effects in the treatment of migraine through agonist effects at the 5-HT1F receptor; however, the precise mechanism is unknown.
12.2. Pharmacodynamics
Cardiac Electrophysiology
At a dose two times the maximum recommended daily dose, REYVOW does not prolong the QTc interval to any clinically relevant extent.
12.3. Pharmacokinetics
Absorption
Following oral administration, lasmiditan is rapidly absorbed with a median tmax of 1.8 hours. In patients with migraine, the absorption or pharmacokinetics of lasmiditan was not different during a migraine attack versus during the interictal period.
Effect of Food
Coadministration of lasmiditan with a high-fat meal increased the mean lasmiditan Cmax and AUC values by 22% and 19%, respectively, and delayed the median tmax by 1 hour. This difference in exposure is not expected to be clinically significant [see Dosage and Administration (2)]. Lasmiditan was administered without regard to food in clinical efficacy studies.
Distribution
The human plasma protein binding of lasmiditan is approximately 55% to 60% and independent of concentration between 15 and 500 ng/mL.
Elimination
Lasmiditan was eliminated with a geometric mean t½ value of approximately 5.7 hours. No accumulation of lasmiditan was observed with daily dosing. Lasmiditan is primarily eliminated via metabolism, with ketone reduction representing the major pathway. Renal excretion is a minor route of lasmiditan clearance.
Metabolism
Lasmiditan undergoes hepatic and extrahepatic metabolism primarily by non-CYP enzymes. The following enzymes are not involved in metabolism of lasmiditan: MAO-A, MAO-B, flavin monooxygenase 3, CYP450 reductase, xanthine oxidase, alcohol dehydrogenase, aldehyde dehydrogenase, and aldo-keto reductases. Lasmiditan is also metabolized to M7 (oxidation on piperidine ring) and M18 (combination of M7 and M8 pathways). These metabolites are considered pharmacologically inactive.
Excretion
Recovery of unchanged lasmiditan in urine was low and accounted for approximately 3% of the dose. Metabolite S-M8 represented approximately 66% of the dose in urine, with the majority of recovery within 48 hours postdose.
Specific Populations
Age, Sex, Race/Ethnicity, and Body Weight
Based on a population pharmacokinetic (PK) analysis, age, sex, race/ethnicity, and body weight did not have a significant effect on the PK (Cmax and AUC) of lasmiditan. Therefore, no dose adjustments are warranted based on age, sex, race/ethnicity, or body weight.
Geriatric Use
In a clinical pharmacology study, administration of lasmiditan to subjects 65 years of age or older demonstrated 26% greater exposure in AUC and 21% higher Cmax, compared to subjects 45 years of age or less. This difference in exposure is not expected to be clinically significant [see Use in Specific Populations (8.5)].
Renal Impairment
In a clinical pharmacology study, administration of lasmiditan to subjects with severe renal impairment (eGFR <30 mL/min/1.73 m²) demonstrated 18% greater exposure in AUC and 13% higher Cmax, compared to subjects with normal renal function. No dose adjustment is required based on renal function.
Hepatic Impairment
In a clinical pharmacology study, subjects with mild and moderate hepatic impairment (Child-Pugh Class A and B, respectively) demonstrated 11% and 35%, respectively, greater exposure [AUC] to lasmiditan, compared to subjects with normal hepatic function. The Cmax were higher by 19% and 33%, respectively, for subjects with mild and moderate hepatic impairment. This difference in exposure is not expected to be clinically significant. The use of lasmiditan has not been studied in subjects with severe hepatic impairment [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Potential for Lasmiditan to Affect Other Drugs
Drug Metabolizing Enzymes:
Lasmiditan is an in-vitro inhibitor of CYP2D6 but did not significantly inhibit the activity of other CYP450 enzymes. Modeling and simulation of the impact of lasmiditan on the exposure of dextromethorphan, a recognized sensitive CYP2D6 substrate, indicate that lasmiditan is unlikely to exert clinically significant inhibition of CYP2D6. Lasmiditan, M7, S-M8, and [S,R]-M18 are not reversible or time-dependent inhibitors of monoamine oxidase A (MAO-A).
Daily dosing of lasmiditan did not alter the PK of midazolam, caffeine, or tolbutamide, which are substrates of CYP3A, CYP1A2, and CYP2C9, respectively. Coadministration of lasmiditan with sumatriptan, propranolol, or topiramate resulted in no clinically meaningful changes in exposure of these medicinal products.
Drug Transporters:
Lasmiditan inhibits P-gp and BCRP in-vitro [see Drug Interactions (7.4)].
Lasmiditan inhibits OCT1 in-vitro. However, in a drug-drug interaction study with lasmiditan and sumatriptan (OCT1 substrate), no change in sumatriptan PK was observed. Lasmiditan inhibits renal efflux transporters, MATE1 and MATE2-K, in-vitro.
Potential for Other Drugs to Affect Lasmiditan
Drug Metabolizing Enzymes:
Lasmiditan undergoes hepatic and extrahepatic metabolism primarily by non-CYP enzymes. Therefore, it is unlikely that CYP inhibitors or inducers will affect lasmiditan pharmacokinetics. Clinical studies of lasmiditan with sumatriptan, propranolol, or topiramate did not show any significant drug interaction potential.
Drug Transporters:
Lasmiditan is a substrate for P-gp in-vitro.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No drug-related tumors were observed following oral administration of lasmiditan to TgRasH2 mice at doses of up to 150 (males) or 250 (females) mg/kg/day for 26 weeks or to rats at doses of up to 75 mg/kg/day for 2 years. Plasma exposures (AUC) at the highest dose tested in rats were approximately 15 times that in humans at the maximum recommended human dose (MRHD) of 200 mg/day.
Mutagenesis
Lasmiditan was negative in in vitro (bacterial reverse mutation, chromosomal aberration in mammalian cells) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
Oral administration of lasmiditan to male (0, 100, 175, or 200 mg/kg/day) or female (0, 100, 150, or 200 mg/kg/day) rats prior to and during mating and continuing in females to Gestation Day 7 resulted in no adverse effects on fertility or reproductive performance. Plasma exposures (AUC) at the highest dose tested (200 mg/kg/day) were approximately 26 times that in humans at the MRHD.
14. Clinical Studies
14.1 Migraine
The efficacy of REYVOW in the acute treatment of migraine was demonstrated in two randomized, double-blind, placebo-controlled trials [Study 1 (NCT02439320) and Study 2 (NCT02605174)]. These studies enrolled patients with a history of migraine with and without aura according to the International Classification of Headache Disorders (ICHD-II) diagnostic criteria. Patients were predominantly female (84%), and White (78%), with a mean age of 42 years (range 18-81). Twenty-two percent of patients were taking preventive medication for migraine at baseline. Study 1 randomized patients to REYVOW 100 mg (n=744), or 200 mg (n=745) or placebo (n=742) and Study 2 randomized patients to REYVOW 50 mg (n=750), 100 mg (n=754), or 200 mg (n=750) or placebo (n=751). Patients were allowed to take a rescue medication 2 hours after taking study drug; however, opioids, barbiturates, triptans, and ergots were not allowed within 24 hours of study drug administration.
The primary efficacy analyses were conducted in patients that treated a migraine with moderate to severe pain within 4 hours of the onset of the attack. The efficacy of REYVOW was established by an effect on pain freedom at 2 hours and Most Bothersome Symptom (MBS) freedom at 2 hours compared to placebo for Studies 1 and 2. Pain freedom was defined as a reduction of moderate or severe headache pain to no pain, and MBS freedom was defined as the absence of the self-identified MBS (photophobia, phonophobia, or nausea). Among patients who selected an MBS, the most commonly selected MBS was photophobia (54%), followed by nausea (24%), and phonophobia (22%).
In both studies, the percentage of patients achieving pain freedom and MBS freedom 2 hours after treatment was significantly greater among patients receiving REYVOW at all doses compared to those receiving placebo (see Table 2).
Table 2. Migraine Efficacy Endpoints after Treatment for Studies 1 and 2:
| Study 1 | Study 2 | ||||||
|---|---|---|---|---|---|---|---|
| REYVOW 100 mg | REYVOW 200 mg | Placebo | REYVOW 50 mg | REYVOW 100 mg | REYVOW 200 mg | Placebo | |
| Pain Free at 2 hours | |||||||
| N | 498 | 503 | 515 | 544 | 523 | 521 | 534 |
| % Responders | 28.3 | 31.8 | 15.3 | 28.3 | 31.4 | 38.8 | 21.0 |
| Difference from placebo (%) | 13 | 16.5 | 7.3 | 10.4 | 17.8 | ||
| p-value | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 | ||
| MBS Free at 2 hours | |||||||
| N | 464 | 467 | 480 | 502 | 491 | 478 | 509 |
| % Responders | 41.2 | 40.7 | 29.6 | 40.8 | 44.0 | 48.7 | 33.2 |
| Difference from placebo (%) | 11.6 | 11.1 | 7.6 | 10.8 | 15.5 | ||
| p-value | <0.001 | <0.001 | 0.014 | <0.001 | <0.001 | ||
Pain relief at 2 hours, defined as a reduction in migraine pain from moderate or severe to mild or none, was also evaluated (see Table 3).
Table 3. Additional Migraine Efficacy Endpoint after Treatment for Studies 1 and 2:
| Study 1 | Study 2 | ||||||
|---|---|---|---|---|---|---|---|
| REYVOW 100 mg | REYVOW 200 mg | Placebo | REYVOW 50 mg | REYVOW 100 mg | REYVOW 200 mg | Placebo | |
| Pain Relief at 2 hoursa | |||||||
| N | 498 | 503 | 515 | 544 | 523 | 521 | 534 |
| % Responders | 54.0 | 55.3 | 40.0 | 55.9 | 61.4 | 61.0 | 45.1 |
| Difference from placebo (%) | 14.0 | 15.3 | 10.8 | 16.3 | 15.9 | ||
a The analysis of pain relief was descriptive and was not controlled for Type I error.
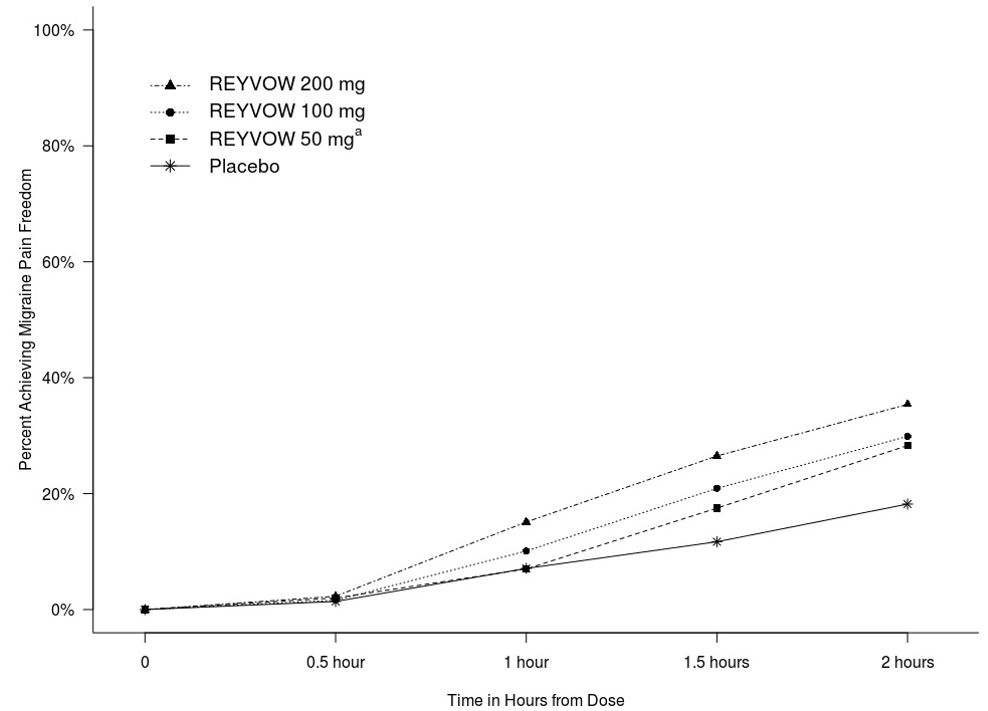
Figure 1 presents the percentage of patients achieving migraine pain freedom within 2 hours following treatment in Studies 1 and 2.
Figure 1. Percentage of Patients Achieving Migraine Pain Freedom within 2 Hours in Pooled Studies 1 and 2:
aThe 50 mg arm was only included in Study 2.
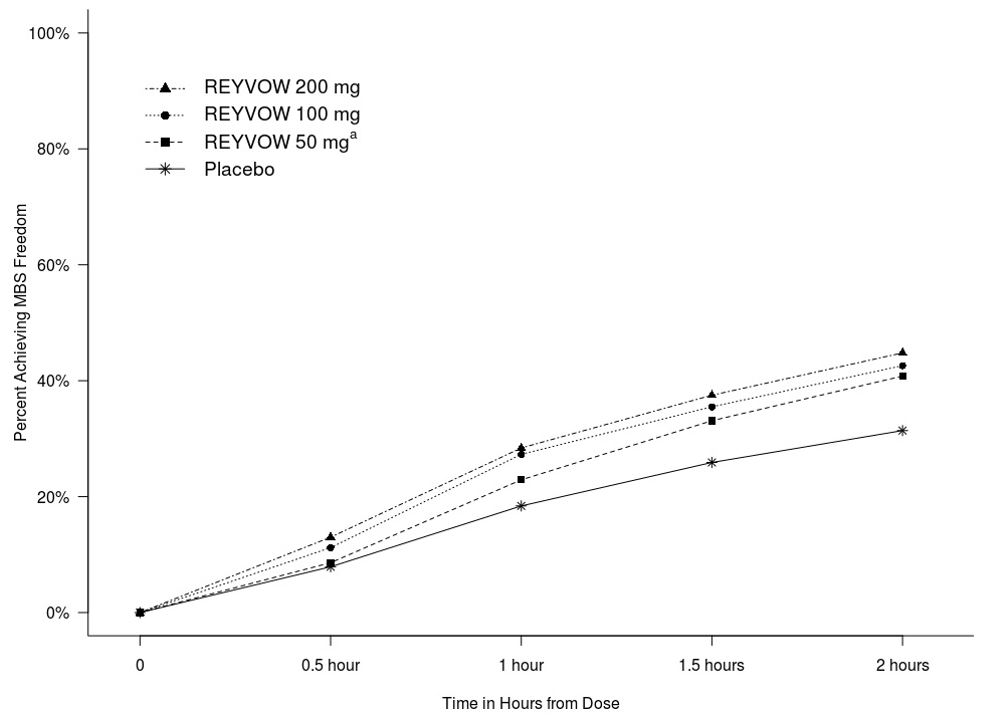
Figure 2 presents the percentage of patients achieving MBS freedom within 2 hours in Studies 1 and 2.
Figure 2. Percentage of Patients Achieving MBS Freedom within 2 Hours in Pooled Studies 1 and 2:
a The 50 mg arm was only included in Study 2.
14.2 Effects on Driving
Driving performance was assessed at 90 minutes after administration of REYVOW 50 mg, 100 mg, 200 mg, alprazolam 1 mg, and placebo in a randomized, double-blind, placebo- and active-controlled, five-period crossover study in 90 healthy volunteers (mean age 34.9 years) using a computer-based driving simulation. Driving performance was evaluated using a validated threshold established in a population with blood alcohol concentration of 0.05%. The primary outcome measure was the difference from placebo in the Standard Deviation of Lateral Position (SDLP), a measure of driving performance. A dose-dependent impairment of computer-based simulated driving performance was seen with all doses of REYVOW at 90 minutes after administration.
Driving performance was also assessed at 8, 12, and 24 hours after administration of REYVOW 100 mg or 200 mg, in a separate randomized, double-blind, placebo- and active-controlled, four-period crossover study in 67 healthy volunteers (mean age 32.8 years) evaluating computer-based simulated driving performance using SDLP as the primary endpoint. Diphenhydramine 50 mg was used as a positive control. The mean SDLP did not reach the threshold for driving impairment at 8 hours or later after administration of REYVOW 100 or 200 mg.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.