RIASTAP Powder for solution for injection or infusion Ref.[9400] Active ingredients: Human fibrinogen
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: CSL Behring GmbH, Emil-von-Behring-Strasse 76, 35041, Marburg, Germany
Therapeutic indications
Treatment of bleeding in patients with congenital hypo-, or afibrinogenaemia with bleeding tendency.
Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of coagulation disorders.
Posology
The dosage and duration of the substitution therapy depend on the severity of the disorder, location and extent of bleeding and the patient’s clinical condition.
The (functional) fibrinogen level should be determined in order to calculate individual dosage and the amount and frequency of administration should be determined on an individual patient basis by regular measurement of plasma fibrinogen level and continuous monitoring of the clinical condition of the patient and other replacement therapies used.
Normal plasma fibrinogen level is in the range of 1.5–4.5 g/l. The critical plasma fibrinogen level below which haemorrhages may occur is approximately 0.5–1.0 g/l.
In case of major surgical intervention, precise monitoring of replacement therapy by coagulation assays is essential.
Initial Dose
If the patient’s fibrinogen level is not known, the recommended dose is 70 mg per kg of body weight (bw) administered intravenously.
Subsequent Dose
The target level (1 g/l) for minor events (e.g. epistaxis, intramuscular bleeding or menorrhagia) should be maintained for at least three days. The target level (1.5 g/l) for major events (e.g. head trauma or intracranial haemorrhage) should be maintained for seven days.
Dose of fibrinogen (mg/kg body weigth) = [Target level (g/l) - measured level (g/l)] / 0.017 (g/l per mg/kg body weigth)
Dosage for neonates, infants and children
Limited data from clinical studies regarding the dosage of Riastap in children are available. Resulting from these studies, as well as from long lasting clinical experience with fibrinogen products, dosage recommendations in the treatment of children are the same as for adults.
Method of Administration
Intravenous infusion or injection.
Riastap should be reconstituted according to section 6.6. The reconstituted solution should be warmed to room or body temperature before administration, then injected or infused slowly at a rate which the patient finds comfortable. The injection or infusion rate should not exceed approximately 5 ml per minute.
Overdose
In order to avoid overdosage, regular monitoring of the plasma level of fibrinogen during therapy is indicated (see 4.2).
In case of overdosage, the risk of development of thromboembolic complications is enhanced.
Shelf life
Shelf life: 5 years.
The physico-chemical stability for the reconstituted product has been demonstrated for 8 hours at room temperature (max. +25°C). From a microbiological point of view the product should be used immediately following reconstitution. If the reconstituted product is not administered immediately, storage shall not exceed 8 hours at room temperature (max. +25°C). The reconstituted product should not be stored in the refrigerator.
Special precautions for storage
Store in a refrigerator (2°C–8°C). Do not freeze. Keep the vial in the outer carton, in order to protect from light.
Nature and contents of container
Vial of colourless glass, Type II Ph. Eur., sealed with a latex-free stopper (bromobutyl rubber), aluminium cap and plastic disc.
Pack with 1 g (Figure 1):
1. One vial containing 1 g human fibrinogen
1. Filter: Pall Syringe Filter
2. Dispensing pin: Mini-Spike Dispensing Pin
Figure 1:
Special precautions for disposal and other handling
General instructions:
- Reconstitution and withdrawal should be carried out under aseptic conditions.
- Reconstituted products should be inspected visually for particulate matter and discoloration prior to administration.
- The solution should be almost colourless to yellowish, clear to slightly opalescent and of neutral pH. Do not use solutions that are cloudy or have deposits.
Reconstitution:
- Warm both the solvent and the powder in unopened vials to room or body temperature (not above 37 °C).
- Riastap should be reconstituted with water for injections (50 ml, not provided).
- Wash hands or use gloves before reconstituting the product.
- Remove the cap from the Riastap vial to expose the central portions of the infusion stoppers.
- Treat the surface of the infusion stopper with antiseptic solution and allow it to dry.
- Transfer the solvent into the vial using an appropriate transfer device. Ensure complete wetting of the powder.
- Gently swirl the vial until the powder is reconstituted and the solution is ready for administration. Avoid strong shaking which causes formation of foam. The powder should be completely reconstituted within 15 minutes (generally 5 to 10 minutes).
- Open the plastic blister containing the dispensing pin (Mini-Spike Dispensing Pin) provided with Riastap (Figure 2).
Figure 2:
- Take the provided dispensing pin and insert it into the stopper of the vial with the reconstituted product (Figure 3).
Figure 3:
- After the dispensing pin is inserted, remove the cap. After the cap is removed, do not touch the exposed surface.
- Open the blister with the filter (Pall Syringe Filter) provided with Riastap (Figure 4).
Figure 4:
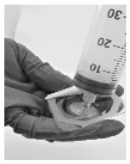
- Screw the syringe onto the filter (Figure 5).
Figure 5:
- Screw the syringe with the mounted filter onto the dispensing pin (Figure 6).
Figure 6:
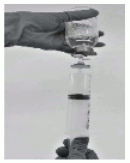
- Draw the reconstituted product into the syringe (Figure 7).
Figure 7:
- When completed, remove the filter, dispensing pin and empty vial from the syringe, dispose of properly, and proceed with administration as usual.
- Reconstituted product should be administered immediately by a separate injection/infusion line.
- Take care that no blood enters syringes filled with product.
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.