RISVAN Suspension fon injection Ref.[109545] Active ingredients: Risperidone
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
The mechanism of action of risperidone, in schizophrenia, is unclear. The drug’s therapeutic activity in schizophrenia could be mediated through a combination of dopamine Type 2 (D2) and serotonin Type 2 (5HT2) receptor antagonism. The clinical effect from risperidone results from the combined concentrations of risperidone and its major metabolite, 9-hydroxyrisperidone (paliperidone) [see Clinical Pharmacology (12.3)]. Antagonism at receptors other than D2 and 5HT2 may explain some of the other effects of risperidone.
12.2. Pharmacodynamics
Risperidone is a monoaminergic antagonist with high affinity (Ki of 0.12 to 7.3 nM) for the serotonin Type 2 (5HT2), dopamine Type 2 (D2), α1 and α2 adrenergic, and H1 histaminergic receptors. Risperidone showed low to moderate affinity (Ki of 47 to 253 nM) for the serotonin 5HT1C, 5HT1D, and 5HT1A receptors, weak affinity (Ki of 620 to 800 nM) for the dopamine D1 and haloperidol-sensitive sigma site, and no affinity (when tested at concentrations > 10-5 M) for cholinergic muscarinic or β1 and β2 adrenergic receptors.
12.3. Pharmacokinetics
Following single doses of RISVAN, plasma exposure (AUC and Cmax) of risperidone, 9-hydroxyrisperidone, and total active moiety (risperidone+9-hydroxyrisperidone) increased in an approximately dose proportional manner over the dose range of 25 mg (0.25 times the maximum recommended dose of RISVAN) to 100 mg. Steady state minimum (Cmin) and peak (Cmax) plasma concentrations of risperidone, 9-hydroxyrisperidone and total active moiety were reached by the first and second injections, respectively. Based on Cmin and Cmax values for total active moiety, the average accumulation ratios were approximately 1.3-fold and 1.8-fold, respectively.
The average plasma concentrations (Cavg) of total active moiety were 22 ng/mL and 28 ng/mL for 3 mg oral risperidone and 75 mg RISVAN, respectively. The Cavg of total active moiety were 29 ng/mL and 37 ng/mL for 4 mg oral risperidone and 100 mg RISVAN, respectively.
Absorption
RISVAN contains risperidone in a suspension delivery system. Following intramuscular (IM) injection, it forms a depot that provides sustained plasma levels over the monthly interval.
Following IM injection, RISVAN shows two absorption peaks for risperidone, 9-hydroxyrisperidone and total active moiety in plasma. The initial peak occurs between 24 h to 48 h and the second peak occurs between Day 18 to Day 25.
Following repeated IM injection of RISVAN 75 mg in the deltoid muscle, on average, 22% higher Cmax was observed compared with injection in the gluteal muscle. The average concentration (Cavg) at steady state was similar for both injection sites.
Distribution
The volume of distribution of risperidone is 1-2 L/kg. In plasma, risperidone is bound to albumin and alpha1-acid glycoprotein. The plasma protein binding of risperidone and 9-hydroxyrisperidone is 90% and 77%, respectively. Neither risperidone nor 9-hydroxyrisperidone displaces each other from plasma binding sites.
Elimination
Metabolism
Risperidone is extensively metabolized in the liver. The main metabolic pathway is through hydroxylation of risperidone to 9-hydroxyrisperidone by the enzyme cytochrome CYP2D6 with minor contribution by CYP3A4. A minor metabolic pathway is through N-dealkylation. The main metabolite, 9-hydroxyrisperidone, has similar pharmacological activity as risperidone. Consequently, the clinical effect of the drug results from the combined concentrations of risperidone plus 9- hydroxyrisperidone.
CYP2D6, is the enzyme responsible for metabolism of many neuroleptics, antidepressants, antiarrhythmics, and other drugs. CYP2D6 is subject to genetic polymorphism (about 6 to 8% of Caucasians, and a very low percentage of Asians, have little or no activity and are “poor metabolizers”) and to inhibition by a variety of substrates and some non-substrates, notably quinidine. Extensive CYP2D6 metabolizers convert risperidone rapidly into 9-hydroxyrisperidone, whereas poor CYP2D6 metabolizers convert it much more slowly. Plasma exposures to total active moiety were similar in CYP2D6 extensive, intermediate and poor metabolizers following intramuscular injection with RISVAN.
Excretion
Risperidone and its metabolites are eliminated via the urine and, to a much lesser extent, via the feces. As illustrated by a mass balance study of a single 1 mg oral dose of 14C-risperidone administered as solution to three healthy male volunteers, total recovery of radioactivity at 1 week was 84%, including 70% in the urine and 14% in the feces.
Following a single injection of RISVAN, the mean half-life (T1/2) ranged from 3.9 to 7.5 days for risperidone, from 8.1 to 8.2 days for 9-hydroxyrisperidone, and from 7.1 to 8.7 days for the active moiety.
Specific Populations
Based on population pharmacokinetic analyses, age, sex and race do not have a clinically meaningful effect on the pharmacokinetics of RISVAN.
Patients with Renal Impairment
RISVAN was not studied in patients with renal impairment, however, in patients with moderate to severe renal disease treated with oral risperidone, the apparent clearance (CL/F) of total active moiety was decreased by 60% in patients with moderate to severe renal disease compared with young healthy subjects [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
RISVAN was not studied in patients with hepatic impairment. In studies with oral risperidone, the pharmacokinetics of subjects with liver disease were comparable to those in young healthy subjects; the mean free fraction of risperidone in plasma was increased by about 35% because of the diminished concentration of both albumin and α1-acid glycoprotein [see Use in Specific Populations (8.7)].
Drug Interaction Studies
No specific drug interaction studies have been performed with RISVAN. The drug interaction data provided in this section is based on studies with oral risperidone. Effects of other drugs on the exposures of risperidone, 9-hydroxyrisperidone and total active moiety as well as the effects of risperidone on the exposures of other drugs are summarized below.
Clinical studies
Effects of Other Drugs on Risperidone, 9-hydroxyrisperidone and Total Active Moiety Pharmacokinetics
Strong CYP2D6 Inhibitors (Fluoxetine and Paroxetine)
Fluoxetine (20 mg once daily) and paroxetine (20 mg once daily), potent CYP2D6 inhibitors, have been shown to increase the plasma concentration of risperidone by 2.5- to 2.8-fold and 3- to 9-fold, respectively. Fluoxetine did not affect the plasma concentration of 9-hydroxyrisperidone. Paroxetine lowered the concentration of 9-hydroxyrisperidone by about 10%. The effects of discontinuation of concomitant fluoxetine or paroxetine therapy on the pharmacokinetics of risperidone and 9-hydroxyrisperidone have not been studied.
Moderate CYP3A4 Inhibitor (Erythromycin)
There were no significant interactions between oral risperidone and erythromycin, a moderate CYP3A4 inhibitor.
Strong CYP3A4 Inducer (Carbamazepine)
Carbamazepine co-administration with oral risperidone decreased the steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone by about 50%. Plasma concentrations of carbamazepine did not appear to be affected. Coadministration of other known CYP3A4 enzyme inducers (e.g., phenytoin, rifampin, and phenobarbital) with risperidone may cause similar decreases in the combined plasma concentrations of risperidone and 9-hydroxyrisperidone.
Amitriptyline, Cimetidine, Ranitidine, Clozapine, Topiramate
Clinically meaningful pharmacokinetic interaction between RISVAN and other drugs, such as amitriptyline, cimetidine, ranitidine and clozapine, is not expected.
- Amitriptyline did not affect the pharmacokinetics of risperidone or of risperidone and 9-hydroxyrisperidone combined following concomitant administration with oral risperidone.
- Cimetidine and ranitidine increased the bioavailability of oral risperidone by 64% and 26%, respectively. However, cimetidine did not affect the AUC of risperidone and 9-hydroxyrisperidone combined, whereas ranitidine increased the AUC of risperidone and 9-hydroxyrisperidone combined by 20%.
- Chronic administration of clozapine with oral risperidone have shown to affect the clearance of risperidone, however, clinical relevance is unknown.
- There was no clinically relevant effect of oral risperidone (1 to 6 mg/day) on the pharmacokinetics of topiramate 400 mg/day.
Effects of Oral Risperidone on Pharmacokinetics of Other Drugs
Lithium
Repeated doses of oral risperidone (3 mg twice daily) did not affect the exposure (AUC) or peak plasma concentrations (Cmax) of lithium (n = 13).
Valproate
Repeated doses of oral risperidone (4 mg once daily) did not affect the pre-dose or average plasma concentrations and exposure (AUC) of valproate (1000 mg/day in three divided doses) compared to placebo (n = 21). However, there was a 20% increase in valproate peak plasma concentration (Cmax) after concomitant administration of oral risperidone.
Topiramate
Oral risperidone administered at doses from 1 to 6 mg/day concomitantly with topiramate 400 mg/day resulted in a 23% decrease in risperidone Cmax and a 33% decrease in risperidone AUC0-12 hour at steady state. Minimal reductions in the exposure to risperidone and 9-hydroxyrisperidone combined, and no change for 9-hydroxyrisperidone were observed. This interaction is unlikely to be of clinical significance. There was no clinically relevant effect of oral risperidone on the pharmacokinetics of topiramate.
Digoxin
Oral risperidone (0.25 mg twice daily) did not show a clinically relevant effect on the pharmacokinetics of digoxin.
CYP2D6 Substrates (Donepezil and Galantamine)
In drug interaction studies, oral risperidone did not significantly affect the pharmacokinetics of donepezil and galantamine, which are metabolized by CYP2D6.
In vitro studies
In vitro studies indicate that risperidone is a relatively weak inhibitor of CYP2D6. Therefore, RISVAN is not expected to substantially inhibit the clearance of drugs that are metabolized by this enzymatic pathway.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity studies were conducted with RISVAN.
Carcinogenicity studies were conducted with oral risperidone in mice and rats. Risperidone was administered in the diet at doses of 0.63, 2.5, and 10 mg/kg for 18-months to mice and for 25-months to rats. These doses are equivalent to approximately 0.2-, 0.75-, and 3-times (mice) and 0.4-, 1.5-, and 6-times (rats) the oral MHRD of 16 mg/day, based on a mg/m² body surface area. A maximum tolerated dose was not achieved in male mice. There were statistically significant increases in pituitary gland adenomas, endocrine pancreas adenomas, and mammary gland adenocarcinomas. The table below summarizes the multiples of the human oral dose on a mg/m² (mg/kg) basis at which these tumors occurred.
Table 6. Summary of Tumor Occurrence at the Multiples of the Human Dose on a mg/m² (mg/kg) Basis with Oral Risperidone Dosing:
| Multiples of Maximum Human Oral Dose in mg/m2 (mg/kg) | ||||
|---|---|---|---|---|
| Tumor Type | Species | Sex | Lowest Effect Level | Highest No-Effect Level |
| Pituitary adenomas | mouse | Female | 0.75 (9.4) | 0.2 (2.4) |
| Endocrine pancreas adenomas | rat | Male | 1.5 (9.4) | 0.4 (2.4) |
| Mammary gland adenocarcinomas | mouse | Female | 0.2 (2.4) | none |
| rat | Female | 0.4 (2.4) | none | |
| rat | Male | 6.0 (37.5) | 1.5 (9.4) | |
| Mammary gland neoplasm, Total | rat | Male | 1.5 (9.4) | 0.4 (2.4) |
Antipsychotic drugs have been shown to chronically elevate prolactin levels in rodents. Serum prolactin levels were not measured during the oral risperidone carcinogenicity studies; however, measurements during subchronic toxicity studies showed that oral risperidone elevated serum prolactin levels 5-to 6-fold in mice and rats at the same doses used in the carcinogenicity studies. Serum prolactin levels increased in a dose-dependent manner up to 6- and 1.5-fold in male and female rats, respectively, at the end of the 24-month treatment with IM risperidone microspheres every 2 weeks. An increase in mammary, pituitary, and endocrine pancreas neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be prolactin-mediated. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unclear [see Warnings and Precautions (5.7)].
Mutagenesis
No evidence of mutagenic or clastogenic potential for risperidone was found in the in vitro tests of Ames gene mutation, the mouse lymphoma assay, rat hepatocyte DNA-repair assay, the chromosomal aberration test in human lymphocytes, Chinese hamster ovary cells, or in the in vivo oral micronucleus test in mice and the sex-linked recessive lethal test in Drosophila.
In addition, no evidence of mutagenic potential was found in the in vitro Ames reverse mutation test for RISVAN.
Impairment of Fertility
No mating and fertility studies were conducted with RISVAN.
Oral risperidone (0.16 to 5 mg/kg) impaired mating, but not fertility, in rat reproductive studies at doses 0.1-to 3-times the oral maximum recommended human dose (MRHD), of 16 mg/day based on mg/m² body surface area. The effect appeared to be in females, since impaired mating behavior was not noted in the male fertility study. In a subchronic study in Beagle dogs in which risperidone was administered orally at doses of 0.31 to 5 mg/kg, sperm motility and concentration were decreased at doses 0.6-to 10-times the oral MRHD based on mg/m² body surface area. Dose-related decreases were also noted in serum testosterone at the same doses. Serum testosterone and sperm parameters partially recovered, but remained decreased after treatment was discontinued. A no-effect dose could not be determined in either rat or dog.
14. Clinical Studies
Efficacy of RISVAN in the treatment of schizophrenia in adults is based upon adequate and well-controlled studies of oral risperidone as well as on one 12-week, randomized, double-blind, placebo-controlled study with RISVAN in adult patients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for schizophrenia (Study 1; NCT 03160521).
Study 1 evaluated the efficacy of RISVAN (75 mg and 100 mg intramuscular every 4 weeks) compared with placebo in adults (age 18 to 65 years, inclusive) experiencing acute exacerbations of schizophrenia. Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score of 80 to 120, inclusive (moderate to severely ill) at the screening visit, occurring 1 to 8 days before the start of double-blind treatment, without an improvement in the PANSS total score of ≥ 20% between screening and the first dosing day.
At the screening visit, patients who had never taken risperidone received 2 mg daily of oral risperidone for 3 days to establish tolerability. Patients were admitted to an inpatient setting, where they remained for 1 to 14 days after the first intramuscular dose of study treatment, as clinically warranted. Patients were randomized to receive 3 doses of intramuscular RISVAN (75 mg or 100 mg) or placebo every 4 weeks (on Day 1, Day 29, and Day 57). No supplemental antipsychotic medications were permitted during the study treatment period.
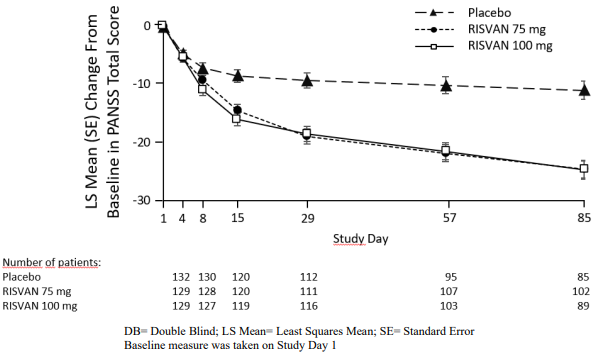
The primary endpoint was the change in PANSS total score from baseline to end of study at Day 85. Both RISVAN 75- and 100-mg doses demonstrated a statistically significant improvement compared with placebo based on the primary endpoint (Table 7). The change in PANSS total score from baseline at each visit from baseline through Day 85 are displayed in Figure 1.
Characteristics of the patient population were balanced across the treatment groups. The mean baseline PANSS total score was approximately 96 in each group. Most patients were male (66% to 68% per group), and the mean ages were 41 to 43 years in each group. Approximately half of the patients were black or African-American (47% to 53% per group) and half were white (46% to 51% per group). A total of 390 patients were included in the primary efficacy population. Subgroup analyses by gender and race did not suggest differences in response. All patients in Study 1 were less than 65 years of age.
Table 7. Primary Efficacy Results for Mean Change from Baseline in PANSS Total Score at Day 85 in Adults with Schizophrenia (Study 1):
| Treatment Group | Number of Patients | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference^a^ (95% CI) |
|---|---|---|---|---|
| RISVAN 75 mg | 129 | 96.3 (8.47) | -24.6 (1.51) | -13.0 (-17.3 to -8.8) |
| RISVAN 100 mg | 129 | 96.1 (8.42) | -24.7 (1.54) | -13.3 (-17.6 to -8.9) |
| Placebo | 132 | 96.4 (7.21) | -11.0 (1.56) | --- |
PANSS: Positive and Negative Syndrome Scale; SD=standard deviation; SE=standard error; LS
Mean=least-squares mean; CI=confidence interval
a Difference (drug minus placebo) in LS mean change from baseline
Figure 1. Change in Baseline in PANSS Total Score by Study Visit in Adults with Schizophrenia (Study 1):
The secondary efficacy endpoint was defined as the mean change from baseline at Day 85 on the Clinical Global Impression – Severity (CGI-S) score. Both RISVAN treatment groups demonstrated statistically significantly better CGI-S scores versus placebo.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.