ROTARIX Powder and solvent for oral suspension Ref.[9867] Active ingredients: Rota virus
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: GlaxoSmithKline Biologicals s.a., Rue de lInstitut 89, B-1330, Rixensart, Belgium
Therapeutic indications
Rotarix is indicated for the active immunisation of infants aged 6 to 24 weeks for prevention of gastroenteritis due to rotavirus infection (see sections 4.2, 4.4 and 5.1).
The use of Rotarix should be based on official recommendations.
Posology and method of administration
Posology
The vaccination course consists of two doses. The first dose may be administered from the age of 6 weeks. There should be an interval of at least 4 weeks between doses. The vaccination course should preferably be given before 16 weeks of age, but must be completed by the age of 24 weeks.
Rotarix may be given with the same posology to preterm infants born after at least 27 weeks of gestational age (see sections 4.8 and 5.1).
In clinical trials, spitting or regurgitation of the vaccine has rarely been observed and, under such circumstances, a replacement dose was not given. However, in the unlikely event that an infant spits out or regurgitates most of the vaccine dose, a single replacement dose may be given at the same vaccination visit.
It is recommended that infants who receive a first dose of Rotarix complete the 2-dose regimen with Rotarix. There are no data on safety, immunogenicity or efficacy when Rotarix is administered for the first dose and another rotavirus vaccine is administered for the second dose or vice versa.
Paediatric population
Rotarix should not be used in children over 24 weeks of age.
Method of administration
Rotarix is for oral use only.
Rotarix should under no circumstances be injected.
For instructions for the preparation or reconstitution of the medicinal product before administration, see section 6.6.
Overdose
Some cases of overdose have been reported. In general, the adverse event profile reported in these cases was similar to that observed after administration of the recommended dose of Rotarix.
Shelf life
3 years.
After reconstitution: After the reconstitution, the vaccine should be administered immediately. If not used immediately, in-use storage should not be longer than 24 hours and at a temperature between 2-25°C.
Special precautions for storage
Store in a refrigerator (2°C–8°C).
Do not freeze.
Store in the original package, in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
1 dose of powder in a glass container (type I glass) with a stopper (rubber butyl).
1 ml of solvent in an oral applicator (type I glass) with a plunger stopper and a protective tip cap (rubber butyl).
Transfer adapter for reconstitution (1/dose) in the following pack sizes:
- pack size of 1 glass container of powder plus 1 oral applicator of solvent
- pack size of 5 glass containers of powder plus 5 oral applicators of solvent
- pack size of 10 glass containers of powder plus 10 oral applicators of solvent
- pack size of 25 glass containers of powder plus 25 oral applicators of solvent
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Before reconstitution
A white deposit and clear supernatant is observed upon storage of the oral applicator containing the solvent. The solvent should be inspected visually for any foreign particulate matter and/or abnormal physical appearance prior to reconstitution.
After reconstitution
The reconstituted vaccine is slightly more turbid than the solvent and is milky white in appearance.
The reconstituted vaccine should also be inspected visually for any foreign particulate matter and/or abnormal physical appearance prior to administration. In the event of either being observed, discard the vaccine.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
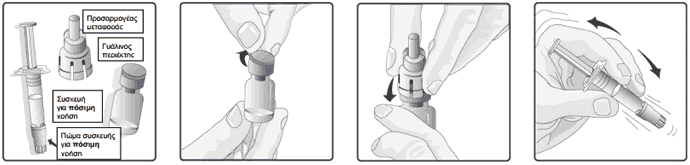
Instructions for reconstitution and administration of the vaccine
1. Remove the plastic cover from the glass container containing the powder.
2. Connect the transfer adapter onto the glass container by pushing it downwards until the transfer adapter is properly and securely placed.
3. Shake the oral applicator containing the solvent vigorously. The shaken suspension will appear as a turbid liquid with a slow settling white deposit.
4. Remove the protective tip cap from the oral applicator.
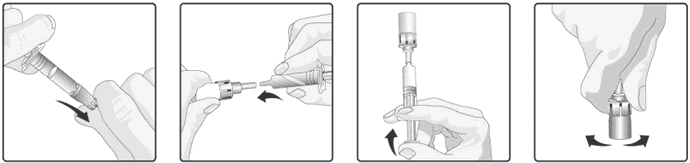
5. Connect the oral applicator into the transfer adapter by pushing it firmly on this device.
6. Transfer the entire content of the oral applicator into the glass container containing the powder.
7. With the oral applicator still attached, shake the glass container and examine it for complete suspension of the powder. The reconstituted vaccine will appear more turbid than the solvent alone. This appearance is normal.
8. Withdraw the entire mixture back into the oral applicator.
9. Remove the oral applicator from the transfer adapter.
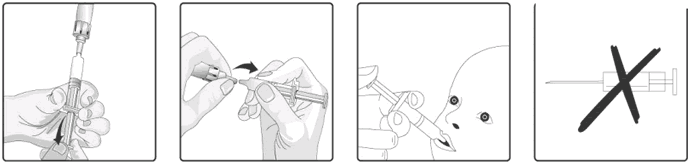
10. This vaccine is for **oral* administration only.* The child should be seated in a reclining position. Administer the entire content of the oral applicator **oral*ly* (by administering the entire content of the oral applicator on the inside of the cheek) Glass container.
11. Do not inject.
If the reconstituted vaccine is to be stored temporarily before administration, replace the protective tip cap on the oral applicator. The oral applicator containing the reconstituted vaccine should be shaken gently again before oral administration. Do not inject.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.