RYZODEG Solution for injection Ref.[50574] Active ingredients: Insulin aspart Insulin degludec
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Novo Nordisk A/S, Novo Allé, DK-2880 Bagsværd, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes. Insulins and analogues for injection, intermediateor long-acting combined with fast-acting
ATC code: A10AD06
Mechanism of action
Insulin degludec and insulin aspart bind specifically to the human insulin receptor and result in the same pharmacological effects as human insulin.
The blood glucose-lowering effect of insulin is due to the facilitated uptake of glucose following the binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose output from the liver.
Pharmacodynamic effects
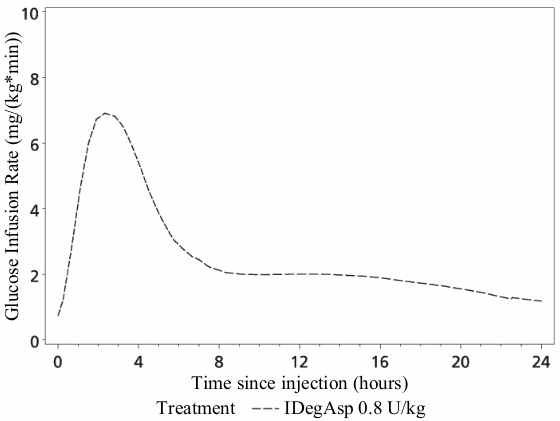
The pharmacodynamic effect of Ryzodeg is distinctively separated for the two components (Figure 1), and the resulting action profile reflects the individual components, the rapid-acting insulin aspart and the basal component insulin degludec.
The basal component of Ryzodeg (insulin degludec) forms soluble multi-hexamers upon subcutaneous injection, resulting in a depot from which insulin degludec is continuously and slowly absorbed into the circulation leading to a flat and stable glucose-lowering effect. This effect is maintained in the co-formulation with insulin aspart and does not interfere with the rapid-acting insulin aspart monomers.
Ryzodeg has a rapid onset of action occurring soon after injection providing mealtime coverage while the basal component has a flat and stable action profile providing continuous coverage of the basal insulin requirements. The duration of action of a single-dose of Ryzodeg is beyond 24 hours.
Figure 1. Pharmacodynamics, single dose – Mean glucose infusion rate profile – Patients with type 1 diabetes – 0.8 U/kg Ryzodeg – Trial 3539:
The total and maximum glucose-lowering effects of Ryzodeg increase linearly with increasing doses. Steady state will occur after 2–3 days of dose administration.
There is no difference in the pharmacodynamic effect of this medicinal product between elderly and younger patients.
Clinical efficacy and safety
Seven multinational, randomised, controlled, open-label, treat-to-target clinical studies of between 26 and 52 weeks' duration were conducted exposing a total of 1,761 patients with diabetes mellitus (1 study involving 362 patients in type 1 diabetes mellitus and 6 studies involving 1,399 patients in type 2 diabetes mellitus) to Ryzodeg. Ryzodeg administered once daily o.d. was compared to insulin glargine (100 units/mL) (IGlar) o.d. in two trials in type 2 diabetes mellitus (Table 1). Ryzodeg b.i.d. was compared to biphasic insulin aspart 30 (BIAsp 30) b.i.d. in two trials in type 2 diabetes mellitus (Table 2) and to insulin degludec (IDeg) o.d. plus insulin aspart (IAsp) 2–4 times daily in one trial in type 2 diabetes mellitus. In one trial in type 2 diabetes mellitus Ryzodeg o.d. was compared to insulin glargine (IGlar) o.d. plus IAsp o.d. After 26 weeks of treatment the Ryzodeg dose could be split into b.i.d. In all trials in type 2 diabetes mellitus, oral antidiabetic drugs (OADs) were allowed. Ryzodeg o.d. plus insulin aspart (IAsp) was also compared to o.d. or b.i.d. insulin detemir (IDet) plus IAsp in type 1 diabetes mellitus (Table 3).
Non-inferiority in HbA1c change from baseline to end-of-trial was confirmed in 6 of the 7 studies against all comparators when treating patients to target, whereas non-inferiority was not confirmed in one study (comparing IDegAsp b.i.d. with IDeg o.d. plus IAsp 2–4 times daily) in type 2 diabetes mellitus.
There is no clinically relevant development of insulin antibodies after long-term treatment of Ryzodeg.
Patients with type 2 diabetes mellitus
In two trials combining insulin and OAD treatment in both insulin-naïve (insulin initiation) and insulin-using (insulin intensification) patients with type 2 diabetes mellitus, Ryzodeg o.d. demonstrated similar glycaemic control (HbA1c) compared to IGlar (administered according to label) (Table 1). As Ryzodeg contains a rapid-acting mealtime insulin (insulin aspart), prandial glycaemic control at the dosing meal is improved relative to administering basal insulin only; see trial results in Table 1. A lower rate of nocturnal hypoglycaemia (defined as episodes between midnight and 6 a.m. confirmed by plasma glucose <3.1 mmol/L or by patient needing third party assistance) was observed with Ryzodeg relative to IGlar (Table 1).
Ryzodeg b.i.d. demonstrated similar glycaemic control (HbA1c) compared with BIAsp 30 b.i.d. in patients with type 2 diabetes mellitus. It demonstrates superior improvements in fasting plasma glucose levels compared to patients treated with BIAsp 30. Ryzodeg causes a lower rate of overall and nocturnal hypoglycaemia (Table 2).
Ryzodeg b.i.d. was compared with IDeg o.d. plus IAsp (2–4 daily injections) in patients with type 2 diabetes mellitus treated with basal insulin in need of treatment intensification with mealtime insulin. The study design included a standardised treatment schedule but allowed for certain adjustments to meet individual needs. Both treatments improved glycaemic control with an estimated mean reduction with Ryzodeg (-1.23%) against IDeg plus IAsp (-1.42%) for the primary endpoint of change from baseline in HbA1c at 26 weeks. This did not meet the pre-specified non-inferiority margin of 0.4% [0.18 (-0.04; 0.41)]. There were no statistically significant differences between the two treatment groups.
In one trial of patients with type 2 diabetes mellitus treated with basal insulin, in need of treatment intensification with mealtime insulin, Ryzodeg o.d. was compared to IGlar o.d. plus IAsp o.d. over 26 weeks. After 26 weeks, the Ryzodeg dose could be split into b.i.d. dosing in the Ryzodeg arm and additional IAsp doses could be administered at other meals (up to 3 times daily) in the IGlar arm. The study design included a standardised treatment schedule but allowed for certain adjustments to meet individual needs. Ryzodeg o.d. demonstrated similar glycaemic control (HbA1c) compared to IGlar o.d. plus IAsp o.d. after 26 weeks (the estimated mean reductions are -1.01% vs -1.09%). Ryzodeg o.d.or b.i.d demonstrated similar glycaemic control (HbA1c) compared to IGlar o.d. plus IAsp 1–3 times daily after 38 weeks (the estimated mean reductions are -1.17% vs -1.26%). Ryzodeg showed a lower rate of nocturnal hypoglycaemia compared to IGlar o.d. plus IAsp during 26 weeks (0.42 vs 0.76 estimated rates per patient year of exposure) and 38 weeks (0.51 vs 0.83 estimated rates per patient year of exposure).
Patients with type 1 diabetes mellitus
In patients with type 1 diabetes mellitus, treatment with Ryzodeg o.d. plus IAsp for the remaining meals demonstrated similar glycaemic control (HbA1c and fasting plasma glucose) with a lower rate of nocturnal hypoglycaemia compared to a basal/bolus regimen with IDet plus IAsp at all meals (Table 3).
There is no clinically relevant development of insulin antibodies after long-term treatment of Ryzodeg.
Table 1. Result from two 26-weeks' trials in type 2 diabetes mellitus with Ryzodeg given once daily:
| Ryzodeg (o.d.)1 Insulin naïve | IGlar (o.d.)1 Insulin naïve | Ryzodeg (o.d.)2 Insulin users | IGlar (o.d.)2 Insulin users | |
|---|---|---|---|---|
| N | 266 | 263 | 230 | 233 |
| Mean HbA1c (%) | ||||
| End of trial Mean change | 7.2 -1.65 | 7.2 -1.72 | 7.3 -0.98 | 7.4 -1.00 |
| Difference: 0.03 [-0.14;0.20] | Difference: -0.03 [-0.20;0.14] | |||
| Fasting Plasma Glucose (FPG) (mmol/L) | ||||
| End of trial Mean change | 6.8 -3.32 | 6.3 -4.02 | 6.3 -1.68 | 6.0 -1.88 |
| Difference: 0.51 [0.09;0.93] | Difference: 0.33 [-0.11;0.77] | |||
| Prandial Blood glucose Increment 90 minutes after dosing meal (Plasma) (mmol/L) | ||||
| End of trial Mean change | 1.9 -1.5 | 3.4 -0.3 | 1.2 -1.5 | 2.6 -0.6 |
| Hypoglycaemia Rate (per patient year of exposure) | ||||
| Severe | 0.01 | 0.01 | 0.00 | 0.04 |
| Confirmed3 | 4.23 | 1.85 | 4.31 | 3.20 |
| Ratio: 2.17 [1.59;2.94] | Ratio: 1.43 [1.07;1.92] | |||
| Nocturnal confirmed3 | 0.19 | 0.46 | 0.82 | 1.01 |
| Ratio: 0.29 [0.13;0.65] | Ratio: 0.80 [0.49;1.30] | |||
1 Once-daily regimen + Metformin
2 Once-daily regimen + Metformin ± pioglitazone ± DPP-4 inhibitor
3 Confirmed hypoglycaemia was defined as episodes confirmed by plasma glucose <3.1 mmol/L or by the patient needing third party assistance. Nocturnal confirmed hypoglycaemia was defined as episodes between midnight and 6 a.m.
Table 2. Result from two 26-weeks' trials in type 2 diabetes mellitus with Ryzodeg given twice daily:
| Ryzodeg (b.i.d.)1 Insulin users | BIAsp 30 (b.i.d.)1 Insulin users | Ryzodeg (b.i.d.)2 Insulin users | BIAsp 30 (b.i.d.)2 Insulin users | |
|---|---|---|---|---|
| N | 224 | 222 | 280 | 142 |
| Mean HbA1c (%) | ||||
| End of trial Mean change | 7.1 -1.28 | 7.1 -1.30 | 7.1 -1.38 | 7.0 -1.42 |
| Difference: -0.03 [-0.18;0.13] | Difference: 0.05 [-0.10;0.20] | |||
| FPG (mmol/L) | ||||
| End of trial Mean change | 5.8 -3.09 | 6.8 -1.76 | 5.4 -2.55 | 6.5 -1.47 |
| Difference: -1.14 [-1.53;-0.76] | Difference: -1.06 [-1.43;-0.70] | |||
| Hypoglycaemia Rate (per patient year of exposure) | ||||
| Severe | 0.09 | 0.25 | 0.05 | 0.03 |
| Confirmed3 | 9.72 | 13.96 | 9.56 | 9.52 |
| Ratio: 0.68 [0.52;0.89] | Ratio: 1.00 [0.76;1.32] | |||
| Nocturnal confirmed3 | 0.74 | 2.53 | 1.11 | 1.55 |
| Ratio: 0.27 [0.18;0.41] | Ratio: 0.67 [0.43;1.06] | |||
1 Twice-daily regimen ± metformin ± pioglitazone ± DPP-4 inhibitor
2 Twice-daily regimen ± metformin
3 Confirmed hypoglycaemia was defined as episodes confirmed by plasma glucose <3.1 mmol/L or by the patient needing third party assistance. Nocturnal confirmed hypoglycaemia was defined as episodes between midnight and 6 a.m.
Table 3. Result of a 26-weeks' trial in type 1 diabetes mellitus with Ryzodeg given once daily:
| Ryzodeg (o.d.)1 | IDet (o.d./b.i.d.)2 | |
|---|---|---|
| N | 366 | 182 |
| Mean HbA1c (%) | ||
| End of trial Mean change | 7.6 -0.73 | 7.6 -0.68 |
| Difference: -0.05 [-0.18;0.08] | ||
| FPG (mmol/L) | ||
| End of trial Mean change | 8.7 -1.61 | 8.6 -2.41 |
| Difference: 0.23 [-0.46;0.91] | ||
| Hypoglycaemia Rate (per patient year of exposure) | ||
| Severe | 0.33 | 0.42 |
| Confirmed3 | 39.2 | 44.3 |
| Ratio: 0.91 [0.76;1.09] | ||
| Nocturnal confirmed3 | 3.71 | 5.72 |
| Ratio: 0.63 [0.49;0.81] | ||
1 Once-daily regimen + insulin aspart to cover mealtime insulin requirements
2 Once- or twice-daily regimen + insulin aspart to cover mealtime insulin requirements
3 Confirmed hypoglycaemia was defined as episodes confirmed by plasma glucose <3.1 mmol/L or by the patient needing third party assistance. Nocturnal confirmed hypoglycaemia was defined as episodes between midnight and 6 a.m.
Cardiovascular safety
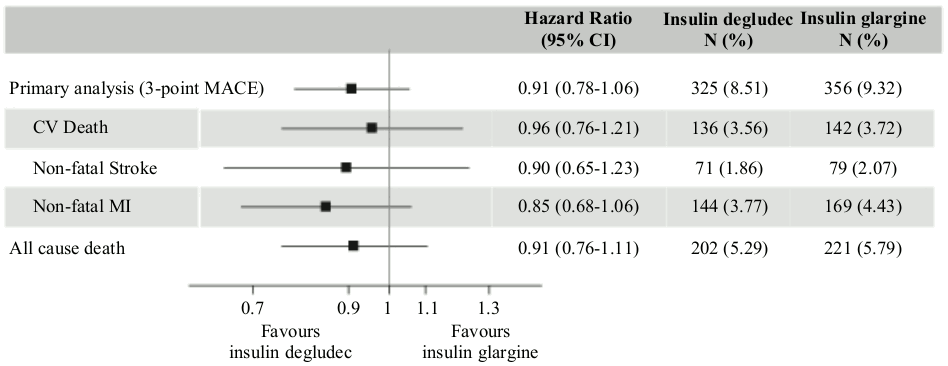
DEVOTE was a randomised, double-blind, and event-driven clinical trial focusing on insulin degludec, the long-acting component of Ryzodeg. The trial had a median duration of 2 years and compared the cardiovascular safety of insulin degludec vs insulin glargine (100 units/mL) in 7,637 patients with type 2 diabetes mellitus at high risk of cardiovascular events.
The primary analysis was time from randomisation to first occurrence of a 3-component major adverse cardiovascular event (MACE) defined as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke. The trial was designed as a non-inferiority trial to exclude a pre-specified risk margin of 1.3 for the hazard ratio (HR) of MACE comparing insulin degludec to insulin glargine. The cardiovascular safety of insulin degludec as compared to insulin glargine was confirmed (HR 0.91 [0.78; 1.06]) (Figure 2).
Results from subgroup analyses (e.g. sex, diabetes duration, CV risk group and previous insulin regimen) were aligned with the primary analysis. At baseline, HbA1c was 8.4% in both treatment groups and after 2 years HbA1c was 7.5% both with insulin degludec and insulin glargine.
Figure 2. Forest plot of analysis of the composite 3-point MACE and individual cardiovascular endpoints in DEVOTE:
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of trials with Ryzodeg in:
- Neonates and infants from birth to less than 12 months of age with type 1 diabetes mellitus.
- In all subsets of the paediatric population in type 2 diabetes mellitus (see section 4.2 for information on paediatric use).
The efficacy and safety of Ryzodeg have been studied in a randomised controlled clinical trial in children and adolescents with diabetes mellitus type 1 for a period of 16 weeks (n=362). Patients in the Ryzodeg arm included 40 exposed children aged 2–5 years, 61 children aged 6–11 years and 80 adolescents aged 12–17 years. Ryzodeg dosed once daily with the main meal plus insulin aspart for the remaining meals showed similar reduction in HbA1c at week 16 and no differences in FPG and SMPG compared to comparator insulin detemir dosed once or twice daily plus mealtime insulin aspart. At week 16, the mean total daily insulin dose was 0.88 vs 1.01 units/kg in the Ryzodeg and insulin detemir arms, respectively. The rates (events per patient-year of exposure) of confirmed hypoglycaemia (ISPAD 2009 definition: 46.23 vs 49.55) and nocturnal confirmed hypoglycaemia (5.77 vs 5.40) were comparable with Ryzodeg vs insulin detemir whereas the rate of severe hypoglycaemia (0.26 vs 0.07) was higher in the Ryzodeg arm although the difference was not statistically significant. Few severe hypoglycaemic episodes were reported in each group; the observed rate of severe hypoglycaemia within the Ryzodeg arm was higher for subjects aged 2–5 years compared to subjects aged 6–11 years or 12–17 years (0.42 vs 0.21 and 0.21 respectively). An efficacy and safety evaluation for adolescent patients with type 2 diabetes mellitus has been made using data from adolescent and adult patients with type 1 diabetes mellitus and adult patients with type 2 diabetes mellitus. This assessment supports the use of Ryzodeg in adolescent patients with type 2 diabetes mellitus.
5.2. Pharmacokinetic properties
Absorption
After subcutaneous injection, soluble and stable multi-hexamers of insulin degludec are formed creating a depot of insulin in the subcutaneous tissue, while not interfering with the rapid release of insulin aspart monomers into the circulation. Insulin degludec monomers gradually separate from the multi-hexamers thus resulting in a slow and continuous delivery of insulin degludec into the circulation. Steady-state serum concentration of the basal component (insulin degludec) is reached after 2–3 days of daily Ryzodeg administration.
The rapid absorption characteristics of the well-established insulin aspart are maintained by Ryzodeg. The pharmacokinetic profile for insulin aspart appears 14 minutes after injection with a peak concentration after 72 minutes.
Distribution
The affinity of insulin degludec to serum albumin corresponds to a plasma protein binding of >99% in human plasma. Insulin aspart has a low binding to plasma proteins (<10%), similar to that seen with regular human insulin.
Biotransformation
Degradation of insulin degludec and insulin aspart is similar to that of human insulin; all metabolites formed are inactive.
Elimination
The half-life after subcutaneous administration of Ryzodeg is determined by the rate of absorption from the subcutaneous tissue. The half-life of the basal component (insulin degludec) at steady state is 25 hours independent of dose.
Linearity
Total exposure with Ryzodeg increases proportionally with increasing dose of the basal component (insulin degludec) and the mealtime component (insulin aspart) in type 1 and type 2 diabetes mellitus.
Gender
There is no gender difference in the pharmacokinetic properties of Ryzodeg.
Elderly, race, renal and hepatic impairment
There are no clinically relevant differences in the pharmacokinetics of Ryzodeg between elderly and younger adult patients, between races or between healthy subjects and patients with renal or hepatic impairment.
Paediatric population
The pharmacokinetic properties of Ryzodeg in type 1 diabetes mellitus were investigated in children (6–11 years) and adolescents (12–18 years) and compared to adults after single dose administration. The steady-state pharmacokinetic properties of the insulin degludec component of Ryzodeg were investigated using a population pharmacokinetic analysis in children down to 1 year of age.
Total exposure and peak concentration of insulin aspart were higher in children than in adults and were similar for adolescents and adults.
The pharmacokinetic properties of insulin degludec in children (1–11 years) and adolescents (12–18 years) were at steady state comparable to those observed in adults with type 1 diabetes mellitus. Total exposure of insulin degludec after single dose administration was, however, higher in children and adolescents than in adults with type 1 diabetes mellitus.
5.3. Preclinical safety data
Non-clinical data reveal no safety concerns for humans based on studies of safety pharmacology, repeated dose toxicity, carcinogenic potential, and toxicity to reproduction.
The ratio of mitogenic relative to metabolic potency for insulin degludec is comparable to that of human insulin.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.