SALBULIN Inhalation powder Ref.[50248] Active ingredients: Salbutamol
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Mylan Products Ltd, Station Close, Potters Bar, Hertfordshire, EN6 1TL, United Kingdom
4.1. Therapeutic indications
Salbulin MDPI Novolizer 100 micrograms is indicated in adults, adolescents and children aged 6 to 12 years.
Salbulin MDPI Novolizer 100 micrograms is recommended for use in patients with reversible airways obstruction such as asthma for relief and prevention of asthma symptoms. It should be used to relieve asthma symptoms when they occur and to prevent symptoms in circumstances known by the patient to precipitate symptoms, for example prior to exercise or allergen exposure.
Salbulin MDPI Novolizer 100 micrograms is particularly useful for the relief of symptoms of asthma, providing it does not delay the introduction and regular use of inhaled corticosteroid therapy.
4.2. Posology and method of administration
Posology
The dose depends on the type, severity, and course of the disorder.
Adults (including older people and adolescents)
For the relief of acute asthma symptoms including bronchospasm a starting dose of one inhalation (100 micrograms) is recommended for adults; this dose may be increased to two inhalations if necessary.
For prevention of exercise-induced or allergen induced symptoms two inhalations (200 micrograms) should be taken 10-15 minutes prior to challenge.
The maximum on-demand use in any 24 hours should not exceed 8 inhalations (equivalent to 800 micrograms).
Children (aged 6 to 12 years)
For the relief of acute asthma symptoms including bronchospasm a starting dose of one inhalation (100 micrograms) is recommended for children aged 6 years and above; this dose may be increased to two inhalations if necessary.
For prevention of exercise-induced or allergen induced symptoms one inhalation (100 micrograms) should be taken 10-15 minutes prior to challenge and a further inhalation (to a total of 200 micrograms), if necessary.
The maximum on-demand use in any 24 hours should not exceed 4 inhalations (equivalent to 400 micrograms).
Children below 6 years of age
Salbulin MDPI Novolizer is not recommended for use in children below age 6 due to insufficient data on safety and efficacy.
For all patients: When other salbutamol inhalers are replaced by Salbulin MDPI Novolizer 100 micrograms, it may be necessary to adjust the dosage regimen as the amount of salbutamol delivered to the lung may vary between different inhalers.
Method of administration
There should be an interval of at least 1 minute between 2 inhalations.
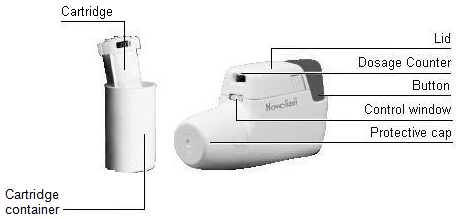
Instructions for using the Novolizer device (Powder inhaler device)
Figure:
Refilling
- Lightly press together the ribbed surface of both sides of the lid, move the lid forwards, and lift off.
- Remove the protective aluminium foil from the cartridge container and take out the new cartridge.
- Insert the cartridge into the Novolizer device with the dosage counter facing the mouthpiece.
- Replace the lid into the side guides from above and push down flat towards the button until it snaps into place. The cartridge can be left in the Novolizer device until it is empty, but it must be replaced with a new cartridge within 6 months of removal from its sealed container.
Note: Salutamol MDPI Novolizer 100 micrograms cartridges may only be used in the Novolizer Powder inhaler.
Usage
- The Novolizer device must be kept horizontal when it is being used.
- Remove the protective cap.
- Prime the Novolizer device by completely depressing the large coloured button. A loud double click will be heard and the colour of the control window will change from red to green. The coloured button should then be released. The colour green in the window indicates that the Novolizer device is primed and ready for use.
- The patient should exhale (not into the powder inhaler).
- The lips should be placed around the mouthpiece and the powder inhaled steadily, deeply and as rapidly as possible (to the maximum inhalation). During this breath a loud click should be heard, indicating that the inhalation was sufficiently strong. The patient should hold their breath for a few seconds and then continue breathing normally. If the patient needs to take more than 1 inhalation, steps 2-5 should be repeated.
- The protective cap should be replaced.
- The number of inhalations left is indicated by the dosage counter.
Note: The large coloured button should only be pressed immediately before inhalation.
It is not possible for the patient to administer a double inhalation in error using the Novolizer device. The clicking sound and the change of colour in the control window indicate that inhalation has been performed correctly. If the colour of the control window does not change then inhalation should be repeated. The Novolizer device cannot be primed a second time unless the inhalation has been performed correctly. If inhalation is not completed correctly after several attempts, then the patient should consult their doctor/physician.
Cleaning
The Novolizer device should be cleaned at regular intervals; at a minimum at least every time the cartridge is changed. Instructions for the patient on how to clean the Novolizer device can be found in the Patient Information Leaflet.
Note: In order to ensure correct use of the Novolizer device, patients should receive thorough instructions on how to use the Novolizer device and their inhaler technique checked to ensure that they are able to use it correctly. Children should only use this product under the supervision of an adult.
4.9. Overdose
Symptoms of an overdose
In the case of an overdose, the above-mentioned undesirable effects (see 4.8, Undesirable effects) occur very quickly and with increased severity. Typical symptoms include: tachycardia, palpitations, arrhythmia, restlessness, sleep disturbances, chest pain and vigorous tremor, especially the hands.
Occasionally, psychotic reactions were observed after excessive doses of salbutamol.
In the case of a salbutamol overdose there can be a shift of potassium into the intracellular space resulting in hypokalaemia, as well as hyperglycaemia, hyperlipidaemia and hyperketonaemia.
Management of an overdose
Treatment after an overdose of salbutamol is mainly symptomatic. The following measures may be considered, depending upon individual circumstances:
- If large amounts of the drug are swallowed, irrigation of the stomach should be considered. Activated charcoal and laxatives may reduce absorption.
- Cardiac symptoms can be treated with a cardioselective beta -blocker. However, attention should be given to an increased risk of bronchospasm in patients with asthma. ECG monitoring is indicated in such patients.
- If blood pressure is markedly reduced, volume substitution (e.g. plasma expanders) is recommended.
- If hypokalaemia develops electrolyte balance should be monitored and electrolytes administered if necessary.
6.3. Shelf life
Medicinal product (Salbutamol inhalation powder) in the container
Shelf life before opening the container: 3 years.
Shelf life after first opening the container: 6 months.
Novolizer Device
Shelf life before first use: 3 years.
In-use shelf life: 1 year or 10 cartridges.
The Novolizer device has been shown to function for at least 2000 metered doses. Therefore a maximum of 10 cartridges containing 200 metered doses can be used with this Novolizer device (within a single year) prior to replacement.
6.4. Special precautions for storage
Do not store above 30°C.
Store in the original package.
When in use Salbulin MDPI Novolizer 100 micrograms should be stored protected from moisture.
6.5. Nature and contents of container
Original sales packs and samples: 1 cartridge containing 200 metered doses filled with not less than 2.308 g of powder packed in a container sealed by aluminium foil and 1 Novolizer device
Refill packs: 1 or 2 cartridges each containing 200 metered doses packed in a container sealed by aluminium foil
Hospital pack: Pack of 10 of original sales packs
All components are made of plastic materials (the cartridge is made of acrylonitrile butadiene styrene (ABS) / polypropylene, the Novolizer device is made of acrylnitrilbutadienestyrol copolymer / polyoxymethylene and the mouthpiece of polycarbonate).
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.