SAXENDA Solution for injection Ref.[50783] Active ingredients: Liraglutide
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Novo Nordisk A/S, Novo Allé, DK-2880 Bagsværd, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, glucagon-like peptide-1 (GLP-1) analogues.
ATC code: A10BJ02
Mechanism of action
Liraglutide is an acylated human glucagon-like peptide-1 (GLP-1) analogue with 97% amino acid sequence homology to endogenous human GLP-1. Liraglutide binds to and activates the GLP-1 receptor (GLP-1R).
GLP-1 is a physiological regulator of appetite and food intake, but the exact mechanism of action is not entirely clear. In animal studies, peripheral administration of liraglutide led to uptake in specific brain regions involved in regulation of appetite, where liraglutide, via specific activation of the GLP1R, increased key satiety and decreased key hunger signals, thereby leading to lower body weight.
GLP-1 receptors are also expressed in specific locations in the heart, vasculature, immune system and kidneys. In mouse models of atherosclerosis, liraglutide prevented aortic plaque progression and reduced inflammation in the plaque. In addition, liraglutide had a beneficial effect on plasma lipids. Liraglutide did not reduce the plaque size of already established plaques.
Pharmacodynamic effects
Liraglutide lowers body weight in humans mainly through loss of fat mass with relative reductions in visceral fat being greater than for subcutaneous fat loss. Liraglutide regulates appetite by increasing feelings of fullness and satiety, while lowering feelings of hunger and prospective food consumption, thereby leading to reduced food intake. Liraglutide does not increase energy expenditure compared to placebo.
Liraglutide stimulates insulin secretion and lowers glucagon secretion in a glucose-dependent manner which results in a lowering of fasting and post-prandial glucose. The glucose-lowering effect is more pronounced in patients with prediabetes and diabetes compared to patients with normoglycaemia. Clinical trials suggest that liraglutide improves and sustains beta-cell function, according to HOMA-B and the proinsulin-to-insulin ratio.
Clinical efficacy and safety
The efficacy and safety of liraglutide for weight management in conjunction with reduced calorie intake and increased physical activity were studied in four phase 3 randomised, double-blind, placebocontrolled trials which included a total of 5,358 adult patients.
- Trial 1 (SCALE Obesity & Pre-Diabetes – 1839): A total of 3,731 patients with obesity (BMI ≥30 kg/m²) or with overweight (BMI ≥27 kg/m²) with dyslipidaemia and/or hypertension were stratified according to prediabetes status at screening and BMI at baseline (≥30 kg/m² or <30 kg/m²). All 3,731 patients were randomised to 56 weeks of treatment and the 2,254 patients with prediabetes at screening were randomised to 160 weeks of treatment. Both treatment periods were followed by a 12-week off drug/placebo observational follow-up period. Lifestyle intervention in the form of an energy-restricted diet and exercise counselling was background therapy for all patients.
The 56-week part of trial 1 assessed body weight loss in all the 3,731 randomised patients (2,590 completers).
The 160-week part of trial 1 assessed time to onset of type 2 diabetes in the 2,254 randomised patients with prediabetes (1,128 completers). - Trial 2 (SCALE Diabetes – 1922): A 56-week trial assessing body weight loss in 846 randomised (628 completers) obese and overweight patients with insufficiently controlled type 2 diabetes mellitus (HbA1c range 7–10%). The background treatment at trial start was either diet and exercise alone, metformin, a sulfonylurea, a glitazone as single agents or any combination hereof.
- Trial 3 (SCALE Sleep Apnoea – 3970): A 32-week trial assessing sleep apnoea severity and body weight loss in 359 randomised (276 completers) obese patients with moderate or severe obstructive sleep apnoea.
- Trial 4 (SCALE Maintenance – 1923): A 56-week trial assessing body weight maintenance and weight loss in 422 randomised (305 completers) obese and overweight patients with hypertension or dyslipidaemia after a preceding weight loss of ≥5% induced by a low-calorie diet.
Body weight
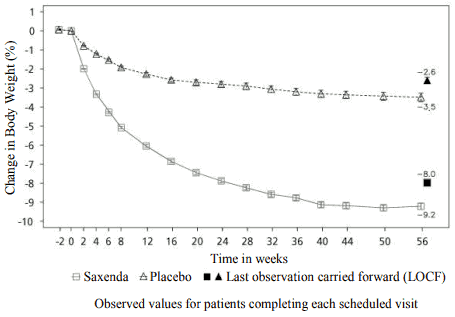
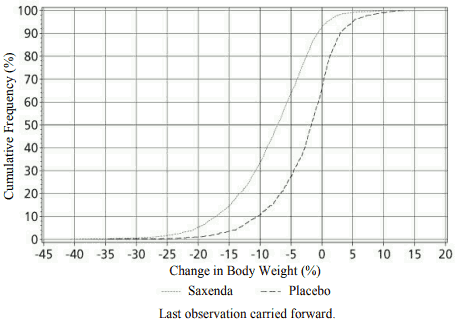
Superior weight loss was achieved with liraglutide compared to placebo in obese/overweight patients in all groups studied. Across the trial populations, greater proportions of the patients achieved ≥5% and >10% weight loss with liraglutide than with placebo (tables 4-6). In the 160-weeks part of trial 1, the weight loss occurred mainly in the first year and was sustained throughout 160 weeks. In trial 4, more patients maintained the weight loss achieved prior to treatment initiation with liraglutide than with placebo (81.4% and 48.9%, respectively). Specific data on weight loss, responders, time course and cumulative distribution of weight change (%) for trials 1-4 are presented in tables 4–8 and figures 1, 2 and 3.
Weight loss response after 12 weeks with liraglutide (3.0 mg) treatment
Early responders were defined as patients who achieved ≥5% weight loss after 12 weeks on treatment dose of liraglutide (4 weeks of dose escalation and 12 weeks on treatment dose). In the 56-week part of trial 1, 67.5% achieved ≥5% weight loss after 12 weeks. In trial 2, 50.4% achieved ≥5% weight loss after 12 weeks. With continued treatment with liraglutide, 86.2% of these early responders are predicted to achieve a weight loss of ≥5% and 51% are predicted to achieve a weight loss of ≥10% after 1 year of treatment. The predicted mean weight loss in early responders who complete 1 year of treatment is 11.2% of their baseline body weight (9.7% for males and 11.6% for females). For patients who have achieved a weight loss of <5% after 12 weeks on treatment dose of liraglutide, the proportion of patients not reaching a weight loss of ≥10% after 1 year is 93.4%.
Glycaemic control
Treatment with liraglutide significantly improved glycaemic parameters across sub-populations with normoglycaemia, prediabetes and type 2 diabetes mellitus. In the 56-week part of trial 1, fewer patients treated with liraglutide had developed type 2 diabetes mellitus compared to patients treated with placebo (0.2% vs. 1.1%). More patients with prediabetes at baseline had reversed their prediabetes compared to patients treated with placebo (69.2% vs. 32.7%). In the 160-week part of trial 1, the primary efficacy endpoint was the proportion of patients with onset of type 2 diabetes mellitus evaluated as time to onset. At week 160, while on treatment, 3% treated with Saxenda and 11% treated with placebo were diagnosed with type 2 diabetes mellitus. The estimated time to onset of type 2 diabetes mellitus for patients treated with liraglutide 3.0 mg was 2.7 times longer (with a 95% confidence interval of [1.9, 3.9]), and the hazard ratio for risk of developing type 2 diabetes mellitus was 0.2 for liraglutide versus placebo.
Cardiometabolic risk factors
Treatment with liraglutide significantly improved systolic blood pressure and waist circumference compared with placebo (tables 4, 5 and 6).
Apnoea-Hypopnoea Index (AHI)
Treatment with liraglutide significantly reduced the severity of obstructive sleep apnoea as assessed by change from baseline in the AHI compared with placebo (table 7).
Table 4 Trial 1: Changes from baseline in body weight, glycaemia and cardiometabolic parameters at week 56:
| Saxenda (N=2437) | Placebo (N=1225) | Saxenda vs. placebo | |||
|---|---|---|---|---|---|
| Body weight | |||||
| Baseline, kg (SD) | 106,3 (21,2) | 106,3 (21,7) | - | ||
| Mean change at week 56, % (95% CI) | -8,0 | -2,6 | -5,4** (-5,8, -5,0) | ||
| Mean change at week 56, kg (95% CI) | -8,4 | -2,8 | -5,6** (-6,0, -5,1) | ||
| Proportion of patients losing ≥5% body weight at week 56, % (95% CI) | 63,5 | 26,6 | 4,8** (4,1, 5,6) | ||
| Proportion of patients losing >10% body weight at week 56, % (95% CI) | 32,8 | 10,1 | 4,3** (3,5, 5,3) | ||
| Glycaemia and cardiometabolic factors | Baseline | Change | Baseline | Change | |
| HbA1c, % | 5,6 | -0,3 | 5,6 | -0,1 | -0,23** (-0,25, -0,21) |
| FPG, mmol/l | 5,3 | -0,4 | 5,3 | -0,01 | -0,38** (-0,42, -0,35) |
| Systolic blood pressure, mmHg | 123,0 | -4,3 | 123,3 | -1,5 | -2,8** (-3,6, -2,1) |
| Diastolic blood pressure, mmHg | 78,7 | -2,7 | 78,9 | -1,8 | -0,9* (-1,4, -0,4) |
| Waist circumference, cm | 115,0 | -8,2 | 114,5 | -4,0 | -4,2** (-4,7, -3,7) |
Full Analysis Set. For body weight, HbA1c, FPG, blood pressure and waist circumference, baseline values are means, changes from baseline at week 56 are estimated means (least-squares) and treatment contrasts at week 56 are estimated treatment differences. For the proportions of patients losing ≥5/>10% body weight, estimated odds ratios are presented. Missing post-baseline values were imputed using the last observation carried forward.
* p <0.05.
** p <0.0001.
CI=confidence interval. FPG=fasting plasma glucose. SD=standard deviation.
Table 5 Trial 1: Changes from baseline in body weight, glycaemia and cardiometabolic:
| Saxenda (N=1472) | Placebo (N=738) | Saxenda vs. placebo | |||
|---|---|---|---|---|---|
| Body weight | |||||
| Baseline, kg (SD) | 107,6 (21,6) | 108,0 (21,8) | - | ||
| Mean change at week 160, % (95% CI) | -6,2 | -1,8 | -4,3** (-4,9, -3,7) | ||
| Mean change at week 160, kg (95% CI) | -6,5 | -2,0 | -4,6** (-5,3, -3,9) | ||
| Proportion of patients losing ≥5% body weight at week 160, % (95% CI) | 49,6 | 23,4 | 3,2** (2,6, 3,9) | ||
| Proportion of patients losing >10% body weight at week 160, % (95% CI) | 24,4 | 9,5 | 3,1** (2,3, 4,1) | ||
| Glycaemia and cardiometabolic factors | Baseline | Change | Baseline | Change | |
| HbA1c, % | 5,8 | -0,4 | 5,7 | -0,1 | -0,21** (-0,24, -0,18) |
| FPG, mmol/l | 5,5 | -0,4 | 5,5 | 0,04 | -0,4** (-0,5, -0,4) |
| Systolic blood pressure, mmHg | 124,8 | -3,2 | 125,0 | -0,4 | -2,8** (-3,8, -1,8) |
| Diastolic blood pressure, mmHg | 79,4 | -2,4 | 79,8 | -1,7 | -0,6 (-1,3, 0,1) |
| Waist circumference, cm | 116,6 | -6,9 | 116,7 | -3,4 | -3,5** (-4,2, -2,8) |
Full Analysis Set. For body weight, HbA1c, FPG, blood pressure and waist circumference, baseline values are means, changes from baseline at week 160 are estimated means (least-squares) and treatment contrasts at week 160 are estimated treatment differences. For the proportions of patients losing ≥5/>10% body weight, estimated odds ratios are presented. Missing post-baseline values were imputed using the last observation carried forward.
** p <0.0001.
CI=confidence interval. FPG=fasting plasma glucose. SD=standard deviation.
Figure 1. Change from baseline in body weight (%) by time in trial 1 (0–56 weeks) parameters at week 160:
Figure 2. Cumulative distribution of weight change (%) after 56 weeks of treatment in trial 1:
Table 6 Trial 2: Changes from baseline in body weight, glycaemia and cardiometabolic parameters at week 56:
| Saxenda (N=412) | Placebo (N=211) | Saxenda vs. placebo | |||
|---|---|---|---|---|---|
| Body weight | |||||
| Baseline, kg (SD) | 105,6 (21,9) | 106,7 (21,2) | - | ||
| Mean change at week 56, % (95% CI) | -5,9 | -2,0 | -4,0** (-4,8, -3,1) | ||
| Mean change at week 56, kg (95% CI) | -6,2 | -2,2 | -4,1** (-5,0, -3,1) | ||
| Proportion of patients losing ≥5% body weight at week 56, % (95% CI) | 49,8 | 13,5 | 6,4** (4,1, 10,0) | ||
| Proportion of patients losing >10% body weight at week 56, % (95% CI) | 22,9 | 4,2 | 6,8** (3,4, 13,8) | ||
| Glycaemia and cardiometabolic factors | Baseline | Change | Baseline | Change | |
| HbA1c, % | 7,9 | -1,3 | 7,9 | -0,4 | -0,9** (-1,1, -0,8) |
| FPG, mmol/l | 8,8 | -1,9 | 8,6 | -0,1 | -1,8** (-2,1, -1,4) |
| Systolic blood pressure, mmHg | 128,9 | -3,0 | 129,2 | -0,4 | -2,6* (-4,6, -0,6) |
| Diastolic blood pressure, mmHg | 79,0 | -1,0 | 79,3 | -0,6 | -0,4 (-1,7, 1,0) |
| Waist circumference, cm | 118,1 | -6,0 | 117,3 | -2,8 | -3,2** (-4,2, -2,2) |
Full Analysis Set. For body weight, HbA1c, FPG, blood pressure and waist circumference, baseline values are means, changes from baseline at week 56 are estimated means (least-squares) and treatment contrasts at week 56 are estimated treatment differences. For the proportions of patients losing ≥5/>10% body weight, estimated odds ratios are presented. Missing post-baseline values were imputed using the last observation carried forward.
* p <0.05.
** p <0.0001.
CI=confidence interval. FPG=fasting plasma glucose. SD=standard deviation.
Table 7 Trial 3: Changes from baseline in body weight and Apnoea-Hypopnoea Index at week 32:
| Saxenda (N=180) | Placebo (N=179) | Saxenda vs. placebo | |||
|---|---|---|---|---|---|
| Σωματικό βάρος | |||||
| Baseline, kg (SD) | 116,5 (23,0) | 118,7 (25,4) | - | ||
| Mean change at week 32, % (95% CI) | -5,7 | -1,6 | -4,2** (-5,2, -3,1) | ||
| Mean change at week 32, kg (95% CI) | -6,8 | -1,8 | -4,9** (-6,2, -3,7) | ||
| Proportion of patients losing ≥5% body weight at week 32, % (95% CI) | 46,4 | 18,1 | 3,9** (2,4, 6,4) | ||
| Proportion of patients losing >10% body weight at week 32 % (95% CI) | 22,4 | 1,5 | 19,0** (5,7, 63,1) | ||
| Baseline | Change | Baseline | Change | ||
| Apnoea-Hypopnoea Index, events/hour | 49,0 | -12,2 | 49,3 | -6,1 | -6,1* (-11,0, -1,2) |
Full Analysis Set. Baseline values are means, changes from baseline at week 32 are estimated means (leastsquares) and treatment contrasts at week 32 are estimated treatment differences (95% CI). For the proportions of patients losing ≥5/>10% body weight, estimated odds ratios are presented. Missing post-baseline values were imputed using the last observation carried forward.
* p<0.05.
** p<0.0001.
CI=confidence interval. SD=standard deviation.
Table 8 Trial 4: Changes from baseline in body weight at week 56:
| Saxenda (N=207) | Placebo (N=206) | Saxenda vs. placebo | |
|---|---|---|---|
| Baseline, kg (SD) | 100,7 (20,8) | 98,9 (21,2) | - |
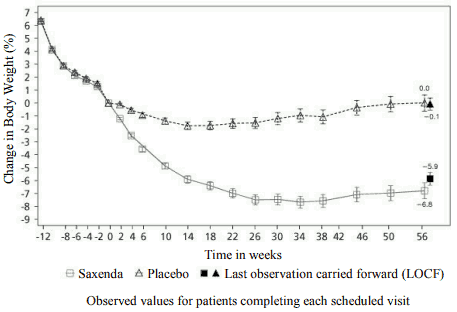
| Mean change at week 56, % (95% CI) | -6,3 | -0,2 | -6,1** (-7,5, -4,6) |
| Mean change at week 56, kg (95% CI) | -6,0 | -0,2 | -5,9** (-7,3, -4,4) |
| Proportion of patients losing ≥5% body weight at week 56, % (95% CI) | 50,7 | 21,3 | 3,8** (2,4, 6,0) |
| Proportion of patients losing >10% body weight at week 56, % (95% CI) | 27,4 | 6,8 | 5,1** (2,7, 9,7) |
Full Analysis Set. Baseline values are means, changes from baseline at week 56 are estimated means (leastsquares) and treatment contrasts at week 56 are estimated treatment differences. For the proportions of patients losing ≥5/>10% body weight, estimated odds ratios are presented. Missing post-baseline values were imputed using the last observation carried forward.
** p <0.0001.
CI=confidence interval. SD=standard deviation.
Figure 3. Change from randomisation (week 0) in body weight (%) by time in trial 4:
Before week 0 patients were only treated with low-calorie diet and exercise. At week 0 patients were randomised to receive either Saxenda or placebo.
Immunogenicity
Consistent with the potentially immunogenic properties of protein and peptide pharmaceuticals, patients may develop anti-liraglutide antibodies following treatment with liraglutide. In clinical trials, 2.5% of patients treated with liraglutide developed anti-liraglutide antibodies. Antibody formation has not been associated with reduced efficacy of liraglutide.
Cardiovascular evaluation
Major adverse cardiovascular events (MACE) were adjudicated by an external independent group of experts and defined as non-fatal myocardial infarction, non-fatal stroke and cardiovascular death. In all the long-term clinical trials with Saxenda, there were 6 MACE for patients treated with liraglutide and 10 MACE for placebo-treated patients. The hazard ratio and 95% CI is 0.33 [0.12; 0.90] for liraglutide versus placebo. A mean increase in heart rate from baseline of 2.5 beats per minute (ranging across trials from 1.6 to 3.6 beats per minute) has been observed with liraglutide in clinical phase 3 trials. The heart rate peaked after approximately 6 weeks. The long-term clinical impact of this mean increase in heart rate has not been established. The change in heart rate was reversible upon discontinuation of liraglutide (see section 4.4).
The Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcomes Results (LEADER) trial included 9,340 patients with insufficiently controlled type 2 diabetes. The vast majority of these had established cardiovascular disease. Patients were randomly allocated to either liraglutide on a daily dose of up to 1.8 mg (4,668) or placebo (4,672), both on a background of standard of care.
The duration of exposure was between 3.5 and 5 years. The mean age was 64 years and the mean BMI was 32.5 kg/m². Mean baseline HbA1c was 8.7 and had improved after 3 years by 1.2 % in patients assigned to liraglutide and by 0.8 % in patients assigned to placebo. The primary endpoint was the time from randomisation to first occurrence of any major adverse cardiovascular events (MACE): cardiovascular death, non-fatal myocardial infarction or non-fatal stroke.
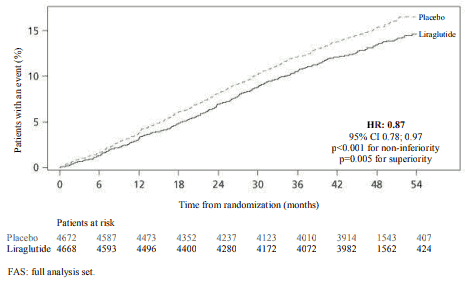
Liraglutide significantly reduced the rate of major adverse cardiovascular events (primary endpoint events, MACE) vs. placebo (3.41 vs. 3.90 per 100 patient years of observation in the liraglutide and placebo groups, respectively) with a risk reduction of 13%, HR 0.87, [0.78, 0.97] [95% CI]) (p=0.005) (see figure 4).
Figure 4. Kaplan Meier plot of time to first MACE – FAS population:
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Saxenda in one or more subsets of the paediatric population in the treatment of obesity (see section 4.2 for information on paediatric use).
In a double-blind trial comparing the efficacy and safety of Saxenda versus placebo on weight loss in adolescent patients aged 12 years and above with obesity, Saxenda was superior to placebo in weight reduction (evaluated as BMI Standard Deviation Score) after 56 weeks of treatment (table 9).
A greater proportion of the patients achieved ≥5% and ≥10% reductions in BMI with liraglutide than with placebo, as well as greater reductions in mean BMI and body weight (table 9). After 26 weeks of off-trial product follow-up period, weight regain was observed with liraglutide vs placebo (table 9).
Table 9 Trial 4180: Changes from baseline in body weight and BMI at week 56 and change in BMI SDS from week 56 to week 82:
| Saxenda (N=125) | Placebo (N=126) | Saxenda vs. placebo | |
|---|---|---|---|
| BMI SDS | |||
| Baseline, SDS BMI (SD) | 3,14 (0,65) | 3,20 (0,77) | |
| Mean change at week 56 (95% CI) | -0,23 | -0,00 | -0,22* (-0,37, -0,08) |
| Week 56, BMI SDS (SD) | 2,88 (0,94) | 3,14 (0,98) | |
| Mean change from week 56 to week 82, BMI SDS (95% CI) | 0,22 | 0,07 | 0,15** (0,07, 0,23) |
| Body weight | |||
| Baseline, kg (SD) | 99,3 (19.7) | 102,2 (21,6) | - |
| Mean change at week 56, % (95% CI) | -2,65 | 2,37 | -5,01** (-7,63, -2,39) |
| Mean change at week 56, kg (95% CI) | -2,26 | 2,25 | -4,50** (-7,17, -1,84) |
| BMI | |||
| Baseline, kg/m² (SD) | 35,3 (5,1) | 35,8 (5,7) | - |
| Mean change at week 56, kg/m² (95% CI) | -1,39 | 0,19 | -1,58** (-2,47, -0,69) |
| Proportion of patients with ≥5% reduction in baseline BMI at week 56, % (95% CI) | 43,25 | 18,73 | 3,31** (1,78, 6,16) |
| Proportion of patients with ≥10% reduction in baseline BMI at week 56, % (95% CI) | 26,08 | 8,11 | 4,00** (1,81, 8,83) |
Full Analysis Set. For BMI SDS, body weight and BMI, baseline values are means, changes from baseline at week 56 are estimated means (least-squares) and treatment contrasts at week 56 are estimated treatment differences. For BMI SDS, value at week 56 are means, changes from week 56 to week 82 are estimated means (least-squares) and treatment contrasts at week 82 are estimated treatment differences. For the proportions of patients losing ≥5%/≥10% baseline BMI, estimated odds ratios are presented. Missing observations were imputed from the placebo arm based on a jump to reference multiple (x100) imputation approach.
* p <0.01,
** p <0.001.
CI=confidence interval. SD=standard deviation.
Based on tolerability, 103 patients (82.4%) escalated and remained on dose of 3.0 mg, 11 patients (8.8%) escalated and remained on dose of 2.4 mg, 4 patients (3.2%) escalated and remained on dose of 1.8 mg, 4 patients (3.2%) escalated and remained on dose of 1.2 mg and 3 patients (2.4%) remained on dose of 0.6 mg.
No effects on growth or pubertal development were found after 56 weeks of treatment.
A 16-week double-blind, 36 week open-label study was conducted to evaluate the efficacy and safety of Saxenda in paediatric patients with Prader-Willi Syndrome and obesity. The study included 32 patients between 12 to <18 years of age (part A) and 24 patients between 6 to <12 years of age (part B). Patients were randomized 2:1 to receive Saxenda or placebo. Patients with a body weight less than 45 kg started dose escalation at a lower dose; 0.3 mg instead of 0.6 mg and were escalated to a maximum dose of 2.4 mg.
The estimated treatment difference in mean BMI SDS at 16 weeks (part A: -0.20 vs -0.13, part B: -0.50 vs -0.44) and 52 weeks (part A: -0.31 vs -0.17, part B: -0.73 vs -0.67) were similar with Saxenda and placebo.
No additional safety concerns were seen in the trial.
5.2. Pharmacokinetic properties
Absorption
The absorption of liraglutide following subcutaneous administration was slow, reaching maximum concentration approximately 11 hours post dosing. The average liraglutide steady state concentration (AUCτ/24) reached approximately 31 nmol/L in obese (BMI 30-40 kg/m²) patients following administration of 3 mg liraglutide. Liraglutide exposure increased proportionally with dose. Absolute bioavailability of liraglutide following subcutaneous administration is approximately 55%.
Distribution
The mean apparent volume of distribution after subcutaneous administration is 20-25 L (for a person weighing approximately 100 kg). Liraglutide is extensively bound to plasma protein (>98%).
Biotransformation
During 24 hours following administration of a single [3H]-liraglutide dose to healthy subjects, the major component in plasma was intact liraglutide. Two minor plasma metabolites were detected (≤9% and ≤5% of total plasma radioactivity exposure).
Elimination
Liraglutide is endogenously metabolised in a similar manner to large proteins without a specific organ as major route of elimination. Following a [3H]-liraglutide dose, intact liraglutide was not detected in urine or faeces. Only a minor part of the administered radioactivity was excreted as liraglutide-related metabolites in urine or faeces (6% and 5%, respectively). The urine and faeces radioactivity was mainly excreted during the first 6-8 days and corresponded to three minor metabolites, respectively.
The mean clearance following subcutaneous administration of liraglutide is approximately 0.9-1.4 L/h with an elimination half-life of approximately 13 hours.
Special populations
Elderly
Age had no clinically relevant effect on the pharmacokinetics of liraglutide based on the results from a population pharmacokinetic analysis of data from overweight and obese patients (18 to 82 years). No dosage adjustment is required based on age.
Gender
Based on the results of population pharmacokinetic analysis, females have 24% lower weight adjusted clearance of liraglutide compared to males. Based on the exposure response data, no dose adjustment is necessary based on gender.
Ethnic origin
Ethnic origin had no clinically relevant effect on the pharmacokinetics of liraglutide based on the results of population pharmacokinetic analysis which included overweight and obese patients of White, Black, Asian and Hispanic/non-Hispanic groups.
Body weight
The exposure of liraglutide decreases with an increase in baseline body weight. The 3.0 mg daily dose of liraglutide provided adequate systemic exposures over the body weight range of 60-234 kg evaluated for exposure response in the clinical trials. Liraglutide exposure was not studied in patients with body weight >234 kg.
Hepatic impairment
The pharmacokinetics of liraglutide was evaluated in patients with varying degree of hepatic impairment in a single-dose trial (0.75 mg). Liraglutide exposure was decreased by 13-23% in patients with mild to moderate hepatic impairment compared to healthy subjects. Exposure was significantly lower (44%) in patients with severe hepatic impairment (Child Pugh score >9).
Renal impairment
Liraglutide exposure was reduced in patients with renal impairment compared to individuals with normal renal function in a single-dose trial (0.75 mg). Liraglutide exposure was lowered by 33%, 14%, 27% and 26%, respectively, in patients with mild (creatinine clearance, CrCl 50-80 ml/min), moderate (CrCl 30-50 ml/min) and severe (CrCl <30 ml/min) renal impairment and in end-stage renal disease requiring dialysis.
Paediatric population
Pharmacokinetic properties for liraglutide 3.0 mg were assessed in clinical studies for adolescent patients with obesity aged 12 to less than 18 years (134 patients, body weight 62-178 kg). The liraglutide exposure in adolescents (age 12 to less than 18 years) was similar to that in adults with obesity.
Pharmacokinetic properties were also assessed in a clinical pharmacology study in the paediatric population with obesity aged 7-11 years (13 patients, body weight 54-87 kg) respectively. Exposure associated with 3.0 mg liraglutide was found to be comparable between the children aged 7 to 11, adolescents and adults with obesity, after correction for body weight.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeat-dose toxicity or genotoxicity.
Non-lethal thyroid C-cell tumours were seen in two-year carcinogenicity studies in rats and mice. In rats, a no observed adverse effect level (NOAEL) was not observed. These tumours were not seen in monkeys treated for 20 months. These findings in rodents are caused by a non-genotoxic, specific GLP-1 receptor-mediated mechanism to which rodents are particularly sensitive. The relevance for humans is likely to be low but cannot be completely excluded. No other treatment-related tumours have been found.
Animal studies did not indicate direct harmful effects with respect to fertility but slightly increased early embryonic deaths at the highest dose. Dosing with liraglutide during mid-gestation caused a reduction in maternal weight and foetal growth with equivocal effects on ribs in rats and skeletal variation in the rabbit. Neonatal growth was reduced in rats while exposed to liraglutide and persisted in the post-weaning period in the high dose group. It is unknown whether the reduced pup growth is caused by reduced pup milk intake due to a direct GLP-1 effect or reduced maternal milk production due to decreased caloric intake.
In juvenile rats, liraglutide caused delayed sexual maturation in both males and females at clinical relevant exposures. These delays had no impact upon fertility and reproductive capacity of either sex, or on the ability of the females to maintain pregnancy.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.