SCANDONEST 3% PLAIN Solution for injection Ref.[50492] Active ingredients: Mepivacaine
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
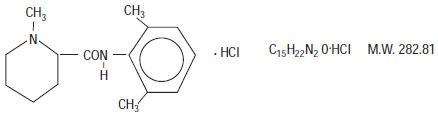
Mepivacaine Hydrochloride, a tertiary amine used as a local anesthetic, is 1-methyl-2', 6'-pipecoloxylidide monohydrochloride with the following structural formula:
It is a white, crystalline, odorless powder soluble in water, but very resistant to both acid and alkaline hydrolysis.
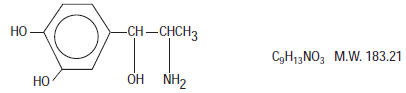
Levonordefrin, a sympathomimetic amine used as a vasoconstrictor in local anesthetic solution, is ( - ) - 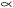
It is a white or buff-colored crystalline solid, freely soluble in aqueous solutions of mineral acids, but practically insoluble in water;
DENTAL CARTRIDGES MAY NOT BE AUTOCLAVED.
SCANDONEST 3% (30 mg/mL) Plain (mepivacaine hydrochloride injection 3% (30 mg/mL)) and SCANDONEST 2% (20 mg/mL) L (mepivacaine hydrochloride 2% (20 mg/mL) with levonordefrin 1:20,000 injection are sterile solutions for injection.
Composition:
| CARTRIDGE | ||
|---|---|---|
| Each mL contains: | 2% | 3% |
| Mepivacaine Hydrochloride | 20 mg | 30 mg |
| Levonordefrin | 0.05 mg | - |
| Sodium Chloride | 4 mg | 6 mg |
| Potassium metabisulfite | 1.2 mg | - |
| Edetate disodium | 0.25 mg | - |
| Sodium Hydroxide q.s. ad pH; Hydrochloric Acid | 0.5 mg | - |
| Water For Injection, qs. ad. | 1 mL | 1 mL |
The pH of the 2% cartridge solution is adjusted between 3.3 and 5.5 with NaOH.
The pH of the 3% cartridge solution is adjusted between 4.5 and 6.8 with NaOH.
| How Supplied |
|---|
|
SCANDONEST 3% (30 mg/mL) Plain; (Mepivacaine Hydrocholoride Injection USP) (NDC 0362-1098-90) is available in cardboard boxes containing 5 blisters of 10 × 1.7 mL single-dose dental cartridges, 50 per carton. SCANDONEST 2% (20 mg/mL) L (Mepivacaine Hydrochloride and Levonordefrin Injection; USP) (NDC 0362-1097-80) is available in cardboard boxes containing 5 blisters of 10 × 1.7 mL single-dose dental cartridges, 50 per carton. Manufactured for SEPTODONT, Inc., 205 Granite Run Dr., Suite 150, Lancaster, PA, USA 17601 by Novocol Pharmaceutical of Canada, Inc., 25 Wolseley Court, Cambridge, Ontario, Canada N1R 6X3 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.