SECONAL Capsule Ref.[27998] Active ingredients: Secobarbital
Source: FDA, National Drug Code (US) Revision Year: 2007
Product description
The barbiturates are nonselective central nervous system (CNS) depressants that are primarily used as sedative hypnotics. In subhypnotic doses, they are also used as anticonvulsants. The barbiturates and their sodium salts are subject to control under the Federal Controlled Substances Act.
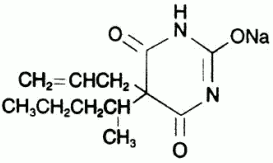
Seconal Sodium (Secobarbital Sodium Capsules, USP) is a barbituric acid derivative and occurs as a white, odorless, bitter powder that is very soluble in water, soluble in alcohol, and practically insoluble in ether. Chemically, the drug is sodium 5-allyl-5-(1-methylbutyl) barbiturate, with the molecular formula C~12~H~17~N~2~NaO~3~. Its molecular weight is 260.27.
The structural formula is as follows:
Each capsule contains 100 mg (0.38 mmol) of secobarbital sodium. It also contains dimethicone, FD&C Red No. 3, FD&C Yellow No. 10, gelatin, magnesium stearate, pregelatinized starch, and titanium dioxide.
| How Supplied |
|---|
|
Seconal Sodium capsules are (orange) and imprinted with RX679 on both the cap and the body: NDC 63304-679-01 100 mg Bottles of 100. Store at 20-25°C (68-77°F) (See USP Controlled Room Temperature). Dispense in a tight container. Manufactured for: Ranbaxy Pharmaceuticals Inc., Jacksonville, FL 32257 USA, by: Ohm Laboratories, Inc., North Brunswick, NJ 08902, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| SECONAL | United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.