SELESYN Oral solution Ref.[49863] Active ingredients: Sodium selenite
Source: Health Products Regulatory Authority (IE) Revision Year: 2016 Publisher: biosyn Arzneimittel GmbH, Schorndorfer Str. 32, D–70734 Fellbach, Germany
4.1. Therapeutic indications
Proven selenium deficiency that cannot be offset from food sources.
4.2. Posology and method of administration
Daily dose
100–200 micrograms selenium (equivalent to 1-2 ampoules). If more selenium is necessary to reach the normal blood level, this dose can be increased to 500 micrograms selenium (equivalent to 5 ampoules = 5 x 100 micrograms or 1 drinking bottle = 500 micrograms, respectively).
Method of administration
Detach a single-dose ampoule from the remainder of the strip of ampoules and open the oral ampoule by twisting the top. Squeeze out the whole of the contents of the ampoule into the mouth.
In the case of the drinking bottles:
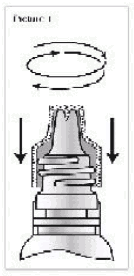
Picture 1:
Picture 2:
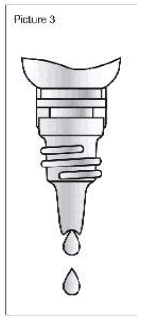
Picture 3:
Picture 1: Before use the drinking bottle has to be made ready for use. For opening the screw cap is first turned clockwise down in order to open the drinking bottle with the integrated spike of the screw cap.
Picture 2: Then the screw cap is turned off anti-clockwise.
Picture 3: The complete contents is placed in the mouth or the required dose is removed by carefully squeezing of the drinking bottle into the enclosed measuring cup with milliliter graduation. Then the drinking bottle is closed firmly.
Hold the liquid in the mouth for about 30–60 seconds before swallowing.
Selenium levels in whole blood or serum should be determined in order to monitor the success of treatment.
There is no time limit to the administration of selesyn in a supplementary dose (100 micrograms selenium per day is equivalent to 1 ampoule).
Dosage in children
2 µg/kg body weight/day at therapy onset and a maintenance dose of 1 µg/kg body weight/day.
For measuring a children's dose of less than 1 ml oral solution approximately 5 ml oral solution is placed in the measuring cup and the needed volume is drawn up with the enclosed pipette. For example for a 1-year-old child with 10 kg body weight the maintenance dose is 10 µg per day corresponding to 0.2 ml oral solution. Selenium levels in whole blood or serum should be determined in order to monitor the success of therapy.
Maximum daily doses for children for a longer time:
| Age (years) | UL (µg selenium/day) |
|---|---|
| 1-3 | 60 |
| 4-6 | 90 |
| 7-10 | 130 |
| 11-14 | 200 |
| 15-17 | 250 |
Dosage in special patient groups
No scientific evidence exists which would require dosage adjustment in patients with renal or hepatic impairment.
Dosage in patients with renal or hepatic impairment
There is no scientific evidence on dosage adjustment in patients with renal or hepatic impairment.
4.9. Overdose
Signs of an acute overdose are an odour of garlic on the breath, tiredness, nausea, diarrhoea and abdominal pain. Chronic overdose can affect growth of nails and hair and may lead to peripheral polyneuropathy.
Countermeasures include gastric lavage, forced diuresis or the administration of high doses of vitamin C. In the case of an extreme overdose (1,000–10,000 times the normal dose) an attempt should be made to eliminate the selenium by dialysis. Administration of dimercaprol is not recommended as the toxic effect of selenium is potentiated.
6.3. Shelf life
Unopened: 30 months.
Use immediately after opening. Discard any unused contents.
6.4. Special precautions for storage
Do not store above 25°C.
6.5. Nature and contents of container
Drinking bottles of plastic (LDPE) each containing 10 ml of oral solution with a PP screw cap, a measuring cup and a dosing pipette.
Pack sizes: 10, 20, 50.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.