SILAPO Solution for injection in pre-filled syringe Ref.[49811] Active ingredients: Epoetin zeta
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: STADA Arzneimittel AG, Stadastrasse 2-18, D-61118 Bad Vilbel, Germany
4.1. Therapeutic indications
Silapo is indicated for the treatment of symptomatic anaemia associated with chronic renal failure (CRF):
- in adults and paediatrics aged 1 to 18 years on haemodialysis and adult patients on peritoneal dialysis (see section 4.4).
- in adults with renal insufficiency not yet undergoing dialysis for the treatment of severe anaemia of renal origin accompanied by clinical symptoms in patients (see section 4.4).
Silapo is indicated in adults receiving chemotherapy for solid tumours, malignant lymphoma or multiple myeloma, and at risk of transfusion as assessed by the patient's general status (e.g. cardiovascular status, pre-existing anaemia at the start of chemotherapy) for the treatment of anaemia and reduction of transfusion requirements.
Silapo is indicated in adults in a predonation programme to increase the yield of autologous blood. Treatment should only be given to patients with moderate anaemia (haemoglobin [Hb] concentration range between 10 and 13 g/dL [6.2 and 8.1 mmol/L], no iron deficiency) if blood saving procedures are not available or insufficient when the scheduled major elective surgery requires a large volume of blood (4 or more units of blood for females or 5 or more units for males).
Silapo is indicated for non-iron deficient adults prior to major elective orthopaedic surgery having a high perceived risk for transfusion complications to reduce exposure to allogeneic blood transfusions. Use should be restricted to patients with moderate anaemia (e.g. haemoglobin concentration range between 10 and 13 g/dL or 6.2 and 8.1 mmol/L) who do not have an autologous predonation programme available and with expected moderate blood loss (900 to 1 800 mL).
Silapo is indicated for the treatment of symptomatic anaemia (haemoglobin concentration of ≤10 g/dL) in adults with low- or intermediate-1-risk primary myelodysplastic syndromes (MDS) who have low serum erythropoietin (<200 mU/mL).
4.2. Posology and method of administration
Treatment with Silapo has to be initiated under the supervision of physicians experienced in the management of patients with above indications.
Posology
All other causes of anaemia (iron, folate or Vitamin B12 deficiency, aluminium intoxication, infection or inflammation, blood loss, haemolysis and bone marrow fibrosis of any origin) should be evaluated and treated prior to initiating therapy with Silapo, and when deciding to increase the dose. In order to ensure optimum response to Silapo, adequate iron stores should be assured and iron supplementation should be administered if necessary (see section 4.4).
Treatment of symptomatic anaemia in adult chronic renal failure patients
Anaemia symptoms and sequelae may vary with age, gender and co-morbid medical conditions; a physician's evaluation of the individual patient's clinical course and condition is necessary.
The recommended desired haemoglobin concentration range is between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L). Silapo should be administered in order to increase haemoglobin to not greater than 12 g/dL (7.5 mmol/L). A rise in haemoglobin of greater than 2 g/dL (1.25 mmol/L) over a four week period should be avoided. If it occurs, appropriate dose adjustment should be made as provided.
Due to intra-patient variability, occasional individual haemoglobin values for a patient above and below the desired haemoglobin concentration range may be observed. Haemoglobin variability should be addressed through dose management, with consideration for the haemoglobin concentration range of 10 g/dL (6.2 mmol/L) to 12 g/dL (7.5 mmol/L).
A sustained haemoglobin level of greater than 12 g/dL (7.5 mmol/L) should be avoided. If the haemoglobin is rising by more than 2 g/dL (1.25 mmol/L) per month, or if the sustained haemoglobin exceeds 12 g/dL (7.5 mmol/L) reduce the Silapo dose by 25%. If the haemoglobin exceeds 13 g/dL (8.1 mmol/L), discontinue therapy until it falls below 12 g/dL (7.5 mmol/L) and then reinstitute Silapo therapy at a dose 25% below the previous dose.
Patients should be monitored closely to ensure that the lowest approved effective dose of Silapo is used to provide adequate control of anaemia and of the symptoms of anaemia whilst maintaining a haemoglobin concentration below or at 12 g/dL (7.5 mmol/L).
Caution should be exercised with escalation of erythropoiesis-stimulating agent (ESA) doses in patients with chronic renal failure. In patients with a poor haemoglobin response to ESA, alternative explanations for the poor response should be considered (see sections 4.4 and 5.1).
Treatment with Silapo is divided into two stages – correction and maintenance phase.
Adult haemodialysis patients
In patients on haemodialysis where intravenous access is readily available, administration by the intravenous route is preferable.
Correction phase
The starting dose is 50 IU/kg, 3 times per week.
If necessary, increase or decrease the dose by 25 IU/kg (3 times per week) until the desired haemoglobin concentration range between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L) is achieved (this should be done in steps of at least four weeks).
Maintenance phase
The recommended total weekly dose is between 75 IU/kg and 300 IU/kg.
Appropriate adjustment of the dose should be made in order to maintain haemoglobin values within the desired concentration range between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L).
Patients with very low initial haemoglobin (<6 g/dL or <3.75 mmol/L) may require higher maintenance doses than patients whose initial anaemia is less severe (>8 g/dL or >5 mmol/L).
Adult patients with renal insufficiency not yet undergoing dialysis
Where intravenous access is not readily available Silapo may be administered subcutaneously.
Correction phase
Starting dose of 50 IU/kg, 3 times per week, followed if necessary by a dosage increase with 25 IU/kg increments (3 times per week) until the desired goal is achieved (this should be done in steps of at least four weeks).
Maintenance phase
During the maintenance phase, Silapo can be administered either 3 times per week, and in the case of subcutaneous administration, once weekly or once every 2 weeks.
Appropriate adjustment of dose and dose intervals should be made in order to maintain haemoglobin values at the desired level: haemoglobin between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L). Extending dose intervals may require an increase in dose.
The maximum dosage should not exceed 150 IU/kg 3 times per week, 240 IU/kg (up to a maximum of 20 000 IU) once weekly, or 480 IU/kg (up to a maximum of 40 000 IU) once every 2 weeks.
Adult peritoneal dialysis patients
Where intravenous access is not readily available Silapo may be administered subcutaneously.
Correction phase
The starting dose is 50 IU/kg, 2 times per week.
Maintenance phase
The recommended maintenance dose is between 25 IU/kg and 50 IU/kg, 2 times per week in 2 equal injections.
Appropriate adjustment of the dose should be made in order to maintain haemoglobin values at the desired level between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L).
Treatment of adult patients with chemotherapy-induced anaemia
Anaemia symptoms and sequelae may vary with age, gender, and overall burden of disease; a physician's evaluation of the individual patient's clinical course and condition is necessary.
Silapo should be administered to patients with anaemia (e.g. haemoglobin concentration ≤10 g/dL [6.2 mmol/L]).
The initial dose is 150 IU/kg subcutaneously, 3 times per week.
Alternatively, Silapo can be administered at an initial dose of 450 IU/kg subcutaneously once weekly.
Appropriate adjustment of the dose should be made in order to maintain haemoglobin concentrations within the desired concentration range between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L).
Due to intra-patient variability, occasional individual haemoglobin concentrations for a patient above and below the desired haemoglobin concentration range may be observed. Haemoglobin variability should be addressed through dose management, with consideration for the desired haemoglobin concentration range between 10 g/dL (6.2 mmol/L) and 12 g/dL (7.5 mmol/L). A sustained haemoglobin concentration of greater than 12 g/dL (7.5 mmol/L) should be avoided; guidance for appropriate dose adjustment for when haemoglobin concentrations exceed 12 g/dL (7.5 mmol/L) are described below.
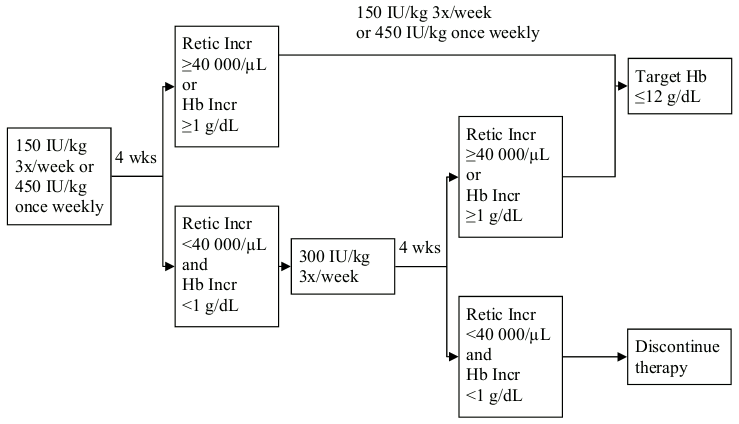
If the haemoglobin concentration has increased by at least 1 g/dL (0.62 mmol/L) or the reticulocyte count has increased ≥40 000 cells/µL above baseline after 4 weeks of treatment, the dose should remain at 150 IU/kg 3 times per week or 450 IU/kg once weekly.If the haemoglobin concentration increase is <1 g/dL (<0.62 mmol/L) and the reticulocyte count has increased <40 000 cells/µl above baseline, increase the dose to 300 IU/kg 3 times per week. If after an additional 4 weeks of therapy at 300 IU/kg 3 times per week, the haemoglobin concentration has increased ≥ 1 g/dL (≥0.62 mmol/L) or the reticulocyte count has increased ≥40 000 cells/µL the dose should remain at 300 IU/kg 3 times per week.If the haemoglobin concentration has increased <1 g/dL (<0.62 mmol/L) and the reticulocyte count has increased <40 000 cells/µL above baseline, response is unlikely and treatment should be discontinued.Dose adjustment to maintain haemoglobin concentrations between 10 g/dL and 12 g/dL (6.2 and 7.5 mmol/L)
If the haemoglobin concentration is increasing by more than 2 g/dL (1.25 mmol/L) per month, or if the haemoglobin concentration level exceeds 12 g/dL (7.5 mmol/L), reduce the Silapo dose by about 25 to 50%.
If the haemoglobin concentration level exceeds 13 g/dL (8.1 mmol/L), discontinue therapy until it falls below 12 g/dL (7.5 mmol/L) and then reinitiate Silapo therapy at a dose 25% below the previous dose.
The recommended dosing regimen is described in the following diagram*:
* 1 g/dL = 0.62 mmol/L; 12 g/dL = 7.5 mmol/L
wks = weeks
Retic Incr = reticulocyte count increase
Patients should be monitored closely to ensure that the lowest approved dose of ESA is used to provide adequate control of the symptoms of anaemia.
Silapo therapy should continue until one month after the end of chemotherapy.
Treatment of adult surgery patients in an autologous predonation programme
Mildly anaemic patients (haematocrit of 33 to 39%) requiring predeposit of ≥4 units of blood should be treated with Silapo 600 IU/kg intravenously, 2 times per week for 3 weeks prior to surgery. Silapo should be administered after the completion of the blood donation procedure.
Treatment of adult patients scheduled for major elective orthopaedic surgery
The recommended dose is Silapo 600 IU/kg administered subcutaneously weekly for three weeks (days -21, -14 and -7) prior to surgery and on the day of surgery.
In cases where there is a medical need to shorten the lead time before surgery to less than three weeks, Silapo 300 IU/kg should be administered subcutaneously daily for 10 consecutive days prior to surgery, on the day of surgery and for four days immediately thereafter.
If the haemoglobin level reaches 15 g/dL (9.38 mmol/L), or higher, during the preoperative period, administration of Silapo should be stopped and further dosages should not be administered.
Treatment of adult patients with low- or intermediate-1-risk MDS
Silapo should be administered to patients with symptomatic anaemia (e.g. haemoglobin concentration ≤10 g/dL (6.2 mmol/L)).
The recommended starting dose is Silapo 450 IU/kg (maximum total dose is 40 000 IU) administered subcutaneously once every week, with not less than 5 days between doses.
Appropriate dose adjustments should be made to maintain haemoglobin concentrations within the target range of 10 g/dL to 12 g/dL (6.2 to 7.5 mmol/L). It is recommended that initial erythroid response be assessed 8 to 12 weeks following initiation of treatment. Dose increases and decreases should be done one dosing step at a time (see diagram below). A haemoglobin concentration of greater than 12 g/dL (7.5 mmol/L) should be avoided.
Dose increase
Dose should not be increased over the maximum of 1 050 IU/kg (total dose 80 000 IU) per week. If the patient loses response or haemoglobin concentration drops by ≥1 g/dL upon dose reduction the dose should be increased by one dosing step. A minimum of 4 weeks should elapse between dose increases.
Dose hold and decrease
Silapo should be withheld when the haemoglobin concentration exceeds 12 g/dL (7.5 mmol/L). Once the haemoglobin level is <11 g/dL the dose can be restarted on the same dosing step or one dosing step down based on physician judgement. Decreasing the dose by one dosing step should be considered if there is a rapid increase in haemoglobin (>2 g/dL over 4 weeks).
Anaemia symptoms and sequelae may vary with age, gender, and co-morbid medical conditions; a physician's evaluation of the individual patient's clinical course and condition is necessary.
Paediatric population
Treatment of symptomatic anaemia in chronic renal failure patients on haemodialysis
Anaemia symptoms and sequelae may vary with age, gender, and co-morbid medical conditions; a physician's evaluation of the individual patient's clinical course and condition is necessary.
In paediatric patients the recommended haemoglobin concentration range is between 9.5 g/dL and 11 g/dL (5.9 and 6.8 mmol/L). Silapo should be administered in order to increase haemoglobin to not greater than 11 g/dL (6.8 mmol/L). A rise in haemoglobin of greater than 2 g/dL (1.25 mmol/L) over a four week period should be avoided. If it occurs, appropriate dose adjustment should be made as provided.
Patients should be monitored closely to ensure that the lowest approved dose of Silapo is used to provide adequate control of anaemia and of the symptoms of anaemia.
Treatment with Silapo is divided into two stages – correction and maintenance phase.
In paediatric patients on haemodialysis where intravenous access is readily available, administration by the intravenous route is preferable.
Correction phase
The starting dose is 50 IU/kg intravenously, 3 times per week.
If necessary, increase or decrease the dose by 25 IU/kg (3 times per week) until the desired haemoglobin concentration range of between 9.5 g/dL and 11 g/dL (5.9 and 6.8 mmol/L) is achieved (this should be done in steps of at least four weeks).
Maintenance phase
Appropriate adjustment of the dose should be made in order to maintain haemoglobin levels within the desired concentration range between 9.5 g/dL and 11 g/dL (5.9 and 6.8 mmol/L).
Generally, children under 30 kg require higher maintenance doses than children over 30 kg and adults. The following maintenance doses were observed in clinical trials after 6 months of treatment.
| Dose (IU/kg given 3 times per week) | ||
|---|---|---|
| Weight (kg) | Median | Usual maintenance dose |
| <10 | 100 | 75-150 |
| 10-30 | 75 | 60-150 |
| >30 | 33 | 30-100 |
Paediatric patients with very low initial haemoglobin (<6.8 g/dL or <4.25 mmol/L) may require higher maintenance doses than patients whose initial haemoglobin is higher (>6.8 g/dL or >4.25 mmol/L).
Anaemia in chronic renal failure patients before initiation of dialysis or on peritoneal dialysis
The safety and efficacy of Silapo in chronic renal failure patients with anaemia before initiation of dialysis or on peritoneal dialysis have not been established. Currently available data for subcutaneous use of epoetin alfa in these populations are described in section 5.1 but no recommendation on posology can be made.
Treatment of paediatric patients with chemotherapy-induced anaemia
The safety and efficacy of Silapo in paediatric patients receiving chemotherapy have not been established (see section 5.1).
Treatment of paediatric surgery patients in an autologous predonation programme
The safety and efficacy of Silapo in paediatrics have not been established. No data are available.
Treatment of paediatric patients scheduled for major elective orthopaedic surgery
The safety and efficacy of Silapo in paediatrics have not been established. No data are available.
Method of administration
Precautions to be taken before handling or administering the medicinal product.
Before use, leave the Silapo syringe to stand until it reaches room temperature. This usually takes between 15 and 30 minutes.
Treatment of symptomatic anaemia in adult chronic renal failure patients
In patients with chronic renal failure where intravenous access is routinely available (haemodialysis patients) administration of Silapo by the intravenous route is preferable.
Where intravenous access is not readily available (patients not yet undergoing dialysis and peritoneal dialysis patients) Silapo may be administered as a subcutaneous injection.
Treatment of adult patients with chemotherapy-induced anaemia
Silapo should be administered as a subcutaneous injection.
Treatment of adult surgery patients in an autologous predonation programme
Silapo should be administered by the intravenous route.
Treatment of adult patients scheduled for major elective orthopaedic surgery
Silapo should be administered as a subcutaneous injection.
Treatment of adult patients with low- or intermediate-1-risk MDS
Silapo should be administered as a subcutaneous injection.
Treatment of symptomatic anaemia in paediatric chronic renal failure patients on haemodialysis
In paediatric patients with chronic renal failure where intravenous access is routinely available (haemodialysis patients) administration of Silapo by the intravenous route is preferable.
Intravenous administration
Administer over at least one to five minutes, depending on the total dose. In haemodialysed patients, a bolus injection may be given during the dialysis session through a suitable venous port in the dialysis line. Alternatively, the injection can be given at the end of the dialysis session via the fistula needle tubing, followed by 10 mL of isotonic saline to rinse the tubing and ensure satisfactory injection of the product into the circulation (see Posology, Adult haemodialysis patients).
A slower administration is preferable in patients who react to the treatment with "flu-like" symptoms (see section 4.8).
Do not administer Silapo by intravenous infusion or in conjunction with other medicinal product solutions (please refer to section 6.6 for further information).
Subcutaneous administration
A maximum volume of 1 mL at one injection site should generally not be exceeded. In case of larger volumes, more than one site should be chosen for the injection.
The injections should be given in the limbs or the anterior abdominal wall.
In those situations in which the physician determines that a patient or caregiver can safely and effectively administer Silapo subcutaneously themselves, instruction as to the proper dosage and administration should be provided.
As with any other injectable product, check that there are no particles in the solution or change in colour.
"Instructions on how to inject Silapo yourself" can be found in section 3 of the package leaflet.
4.9. Overdose
The therapeutic margin of erythropoietin is very wide. Overdosage of erythropoietin may produce effects that are extensions of the pharmacological effects of the hormone. Phlebotomy may be performed if excessively high haemoglobin levels occur. Additional supportive care should be provided as necessary.
6.3. Shelf life
30 months.
6.4. Special precautions for storage
Store in a refrigerator (2°C to 8°C). This temperature range should be closely maintained until administration to the patient.
Keep the pre-filled syringe in the outer carton in order to protect from light.
For the purpose of ambulatory use, the medicinal product may be taken out of the refrigerator, without being replaced, for a maximum period of 3 days at a temperature not above 25 °C. If the medicinal product has not been used at the end of this period, it should be disposed of.
Do not freeze or shake.
6.5. Nature and contents of container
Silapo 1 000 IU/0.3 mL solution for injection in pre-filled syringe:
0.3 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 2 000 IU/0.6 mL solution for injection in pre-filled syringe:
0.6 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 3 000 IU/0.9 mL solution for injection in pre-filled syringe:
0.9 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 4 000 IU/0.4 mL solution for injection in pre-filled syringe:
0.4 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 5 000 IU/0.5 mL solution for injection in pre-filled syringe:
0.5 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 6 000 IU/0.6 mL solution for injection in pre-filled syringe:
0.6 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 8 000 IU/0.8 mL solution for injection in pre-filled syringe:
0.8 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 10 000 IU/1 mL solution for injection in pre-filled syringe:
1 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1 or 6 pre-filled syringes.
Silapo 20 000 IU/0.5 mL solution for injection in pre-filled syringe:
0.5 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1, 4 or 6 pre-filled syringes.
Silapo 30 000 IU/0.75 mL solution for injection in pre-filled syringe:
0.75 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1, 4 or 6 pre-filled syringes.
Silapo 40 000 IU/1 mL solution for injection in pre-filled syringe:
1 mL solution for injection in pre-filled syringe Type I glass with a fixed steel injection needle and a plunger stopper with PTFE coating with or without a needle guard device.
Each pack contains 1, 4 or 6 pre-filled syringes.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Silapo should not be used and discarded
- if the seal is broken,
- if the liquid is coloured or you can see particles floating in it,
- if any liquid has leaked out of the pre-filled syringe or condensation is visible within the sealed blister,
- if you know, or think that it may have been accidentally frozen, or
- if there has been a refrigerator failure.
This medicinal product is for single use only. Only take one dose of Silapo from each syringe.
Do not shake.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.