SOGROYA Solution for injection Ref.[27982] Active ingredients: Somapacitan
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Novo Nordisk A/S, Novo Allé, DK-2880 Bagsværd, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Pituitary and hypothalamic hormones and analogues, somatropin and somatropin agonists
ATC code: H01AC07
Mechanism of action
Somapacitan is a long-acting recombinant human growth hormone derivative. It consists of 191 amino acids similar to endogenous human growth hormone, with a single substitution in the amino acid backbone (L101C) to which an albumin binding moiety has been attached. The albumin binding moiety (side-chain) consists of a fatty acid moiety and a hydrophilic spacer attached to position 101 of the protein.
The mechanism of action of somapacitan is either directly via the GH-receptor and/or indirectly via IGF-I produced in tissues throughout the body, but predominantly by the liver. When growth hormone deficiency is treated with somapacitan a normalisation of body composition (i.e., decreased body fat mass, increased lean body mass) and of metabolic action is achieved.
Pharmacodynamic effects
IGF-I
IGF-I is a generally accepted biomarker for efficacy in AGHD. A dose-dependent IGF-I response is induced following somapacitan administration in AGHD patients. A steady state pattern in IGF-I responses is reached after 1-2 weekly doses.
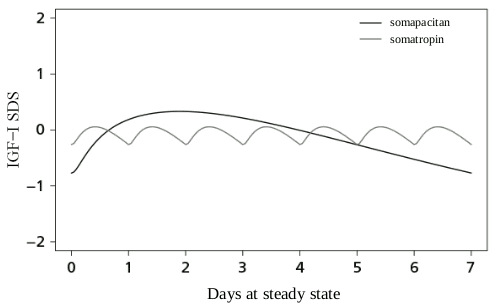
The IGF-I levels fluctuate during the week. The IGF-I response is maximal after 2 to 4 days. Compared with daily GH treatment, the IGF-I profile of somapacitan differs, see Figure 1.
Figure 1. Model-derived IGF-I profiles during steady state of somapacitan and somatropin:
Clinical efficacy and safety
In a 34-week placebo-controlled (double-blind) and active-controlled (open) trial, 301 treatment-naïve adult patients with GHD were randomised (2:1:2) and exposed to once-weekly somapacitan or to placebo or to daily somatropin for a 34-week treatment period (main phase of the trial). The patient population had a mean age of 45.1 years (range 23-77 years; 41 patients were 65 years or above), 51.7% were females, and 69.7% had adult onset GHD.
A total of 272 AGHD patients who completed the 34-week main phase continued in a 53-week open-label extension period. Subjects on placebo were switched to somapacitan and patients on somatropin were re-randomised (1:1) to either somapacitan or somatropin.
Observed clinical effects for the main endpoints in the main treatment phase (Table 3) and extension treatment phase (Table 4) are presented below.
Table 3. Results at 34 weeks:
| Change from baseline at 34 weeksa | somapacitan | somatropin | placebo | Difference somapacitan - placebo [95% CI] p-value | Difference somapacitan - somatropin [95% CI] |
|---|---|---|---|---|---|
| Number of subjects (Ν) | 120 | 119 | 61 | ||
| Truncal fat % (Primary endpoint) | -1.06 | -2.23 | 0.47 | -1.53 [-2.68; -0.38] 0.0090b | 1.17 [0.23; 2.11] |
| Visceral adipose tissue (cm²) | -10 | -9 | 3 | -14 [-21; -7] | -1 [-7; 4] |
| Appendicular skeletal muscle mass (g) | 558 | 462 | -121 | 679 [340; 1.019] | 96 [-182; 374] |
| Lean body mass (g) | 1.394 | 1.345 | 250 | 1144 [459; 1.829] | 49 [-513; 610] |
| IGF-I SDS level | 2.40 | 2.37 | -0.01 | 2.40 [2.09; 2.72] | 0.02 [-0.23; 0.28] |
Abbreviations: N = Number of subjects in full analysis set, CI = Confidence interval, DM=Diabetes mellitus. IGF-I SDS: Insulin-like growth factor-I standard deviation score.
a Body composition parameters are based on dual-energy X-ray absorptiometry (DXA) scanning.
b The primary analysis was a comparison of changes from baseline for somapacitan and placebo in truncal fat %.
Changes in truncal fat % from baseline to the 34 week’s measurements was analysed using an analysis of covariance model with treatment, GHD onset type, sex, region, DM and sex by region by DM interaction as factors and baseline as a covariate incorporating a multiple imputation technique where missing week 34 values were imputed based on data from the placebo group.
Post-hoc subgroup analysis of changes from baseline in truncal fat percentage () compared to placebo at week 34 showed an estimated treatment difference (somapacitan-placebo) of -2.49 [-4.19; -0.79] in men, -0.80% [-2.99; 1.39] in women not on oral oestrogen, -1.44% [-3.97; 1.09] in women on oral oestrogen.
Table 4. Results at 87 weeks:
| Change from baseline at 87 weeksa | somapacitan/ somapacitan | somatropin/ somatropin | placebo/ somapacitan | somatropin/somatropin | Difference somapacitan/ somapacitan vs somatropin/somatropin [95% CI] |
|---|---|---|---|---|---|
| Number of subjects (Ν) | 114 | 52 | 54 | 51 | |
| Truncal fat % | -1.51 | -2.67 | -2.28 | -1.35 | 1.15 [-0.10; 2.40] |
| Visceral adipose tissue (cm²) | -6.64 | -6.85 | -10.21 | -8.77 | 0.22 [-10; 10] |
| Appendicular skeletal muscle mass (g) | 546.11 | 449.09 | 411.05 | 575.80 | 97.02 [-362; 556] |
| Lean body mass (g) | 1,739.05 | 1,305.73 | 1,660.56 | 1,707.82 | 433.32 [-404; 1.271] |
a Body composition parameters are based on DXA scanning.
Observed and simulated IGF-I SDS levels in the clinical study
In the main phase of the clinical study IGF-I SDS values of 0 and above were overall achieved in 53% of somapacitan-treated AGHD study patients after an 8-week dose titration period. This proportion was however lower in particular subgroups such as women on oral oestrogen (32%) and patients with childhood-onset (39%) (Table 5). Post-hoc simulation analyses indicated that the proportions of AGHD patients achieving IGF-I SDS levels above 0 are expected to be higher in case somapacitan dose titration beyond 8 weeks would be allowed. In this simulation analysis, it was assumed that somapacitan dose titration was well-tolerated in all patients until the IGF-I SDS target range or a somapacitan dose of 8 mg per week would be achieved.
Table 5. Proportions of somapacitan-treated AGHD patients with IGF-I SDS levels above 0:
| Subgroups | Men | Women not on oral oestrogen | Women on oral oestrogen | Childhood- onset AGHD | Adult- onset AGHD | All |
|---|---|---|---|---|---|---|
| Observeda | 71% | 46% | 32% | 39% | 60% | 53% |
| Post-hoc simulations | 100% | 96% | 70% | 84% | 92% | 90% |
a The trial was designed to titrate towards a IGF-I SDS level above -0.5
Maintenance dose
Maintenance dose varies from person to person and between male and female patients. The average somapacitan maintenance dose observed in the phase 3 clinical trials was 2.4 mg/week.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Sogroya in all subsets of the paediatric population in growth hormone deficiency (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Somapacitan has pharmacokinetic properties compatible with once weekly administration. The reversible binding to endogenous albumin delays elimination of somapacitan and thereby prolongs the in vivo half-life and duration of action.
The pharmacokinetics of somapacitan following subcutaneous administration have been investigated at dose levels from 0.01 to 0.32 mg/kg in healthy adults, and in doses up to 0.12 mg/kg in adults with GHD.
Overall, somapacitan displays non-linear pharmacokinetics, but in the clinically relevant dose range of somapacitan in adults with GHD, somapacitan pharmacokinetics are approximately linear.
Absorption
In adult patients with GHD median tmax ranged from 4 to 24 hours at doses from 0.02 mg/kg/week to 0.12 mg/kg/week.
Steady state exposure was achieved following 1-2 weekly administration.
Absolute bioavailability of somapacitan in humans has not been investigated.
Distribution
Somapacitan is extensively bound (>99%) to plasma proteins and is expected to be distributed like albumin. Based on population PK analyses, the estimated volume of distribution (V/F) was 14.6 L.
Elimination
The terminal half-life was estimated with geometric means ranging from approximately 2 to 3 days at steady state in AGHD patients (doses: 0.02 to 0.12 mg/kg).
Somapacitan will be present in circulation for approximately 2 weeks after the last dose. Little to no accumulation (mean accumulation ratio: 1-2) of somapacitan following multiple dosing has been observed in AGHD patients.
Biotransformation
Somapacitan is extensively metabolised by proteolytic degradation and cleavage of the linker sequence between the peptide and albumin binder.
Somapacitan was extensively metabolised before excretion and no intact somapacitan was found neither in urine, which was the main excretion route (81%), nor in faeces where 13% of somapacitan related material was found, indicating full biotransformation before excretion.
Special populations
Age
Subjects older than 60 years have higher exposure (29%) than younger subjects at the same somapacitan dose. A lower starting dose for subjects above 60 years is described in section 4.2.
Gender
Female subjects and in particular female subjects on oral oestrogen, have lower exposure (53% for females on oral oestrogen and 30% for females not on oral oestrogen) than male subjects at the same somapacitan dose. A higher starting dose for females on oral oestrogen is described in section 4.2.
Race
There was no difference in somapacitan exposure and IGF-I response between Japanese and White subjects. Despite a higher exposure in Asian Non-Japanese compared to White at the same somapacitan dose, White, Japanese and Asian Non-Japanese needed the same doses to reach similar IGF-I levels. Therefore, there is no dose adjustment recommendation based on race.
Ethnicity
Ethnicity (Hispanic or Latino 4.5% (15 subjects received somapacitan)) was not investigated due to small sample size in the development programme.
Body weight
Despite a higher exposure in subjects with low body weight as compared to subjects with high body weight at the same somapacitan dose, subjects needed the same doses to reach similar IGF-I levels across the body weight range 35 kg to 150 kg. Therefore, there is no dose adjustment recommendation based on body weight.
Renal impairment
A somapacitan dose of 0.08 mg/kg at steady state resulted in higher exposures in subjects with renal impairment, most pronounced in subjects with severe renal impairment and in subjects requiring haemodialysis, where AUC0-168h ratios to normal renal function were 1.75 and 1.63, respectively. In general, somapacitan exposure tended to increase with decreasing GFR.
Higher IGF-I AUC0-168h levels were observed in subjects with moderate and severe renal impairment and subjects requiring haemodialysis, with ratios to normal renal function of 1.35, 1.40 and 1.24 respectively.
Due to the modest increase observed in IGF-I combined with the low recommended starting doses and the individual dose titration of somapacitan, there is no dose adjustment recommendation in patients with renal impairment.
Hepatic impairment
A somapacitan dose of 0.08 mg/kg at steady state resulted in higher exposure in subjects with moderate hepatic impairment with ratios to normal hepatic function of 4.69 for AUC0-168h and 3.52 for Cmax.
Lower somapacitan stimulated IGF-I levels were observed in subjects with mild and moderate hepatic impairment compared to subjects with normal hepatic function (ratio to normal was 0.85 for mild and 0.75 for moderate).
Due to the modest decrease observed in IGF-I combined with the individual dose titration of somapacitan, there is no dose adjustment recommendation in patients with hepatic impairment.
5.3. Preclinical safety data
Preclinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeat-dose toxicity, genotoxicity or pre- and postnatal development.
No carcinogenicity studies have been performed with somapacitan.
No adverse effects were observed on male and female fertility in rats at a dose resulting in exposure at least 13 and 15-times greater than the expected maximum clinical exposure at 8 mg/week for males and females, respectively. However, irregular female oestrus cycle was seen at all doses treated. No evidence of foetal harm was identified when pregnant rats and rabbits were administered subcutaneous somapacitan during organogenesis at doses leading to exposures well above expected exposure at the maximum clinical dose of 8 mg/week (at least 18-fold). At high doses leading to exposure at least 130-fold above the expected maximum clinical exposure at 8 mg/week, short/bent/thickened long bones were found in pups from female rats receiving somapacitan. Such findings in rats are known to resolve after birth and should be regarded as minor malformations, not permanent abnormalities.
Foetal growth was reduced when pregnant rabbits were dosed with somapacitan subcutaneously at exposures at least 9-fold above the expected exposure at the maximum clinical dose of 8 mg/week.
In lactating rats, somapacitan related material was secreted into milk but to a lower level than observed in plasma (up to 50% of level in plasma).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.