SPINRAZA Solution for injection Ref.[9367] Active ingredients: Nusinersen
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Biogen Netherlands B.V., Prins Mauritslaan 13, 1171 LP Badhoevedorp, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Other drugs for disorders of the musculo-skeletal system
ATC code: M09AX07
Mechanism of action
Nusinersen is an antisense oligonucleotide (ASO) which increases the proportion of exon 7 inclusion in survival motor neuron 2 (SMN2) messenger ribonucleic acid (mRNA) transcripts by binding to an intronic splice silencing site (ISS-N1) found in intron 7 of the SMN2 pre-messenger ribonucleic acid (pre-mRNA). By binding, the ASO displaces splicing factors, which normally suppress splicing. Displacement of these factors leads to retention of exon 7 in the SMN2 mRNA and hence when SMN2 mRNA is produced, it can be translated into the functional full length SMN protein.
SMA is a progressive neuromuscular disease resulting from mutations in chromosome 5q in the SMN1 gene. A second gene SMN2, located near SMN1, is responsible for a small amount of SMN protein production. SMA is a clinical spectrum of disease with disease severity linked to fewer numbers of SMN2 gene copies and younger age of symptom onset.
Immunogenicity
The immunogenic response to nusinersen was evaluated in 342 patients with post-baseline plasma samples for ADAs. Overall, 36 Spinraza-treated patients (11%) developed treatment-emergent ADAs, of which 14 (4%) were transient and 22 (6%) were persistent. No discernible effects of ADAs on efficacy or safety have been observed as measured by incidence of AEs including hypersensitivity, anaphylactic reaction, and angio-oedema.
Clinical efficacy and safety
Symptomatic patients
Infantile onset
Study CS3B (ENDEAR) was a Phase 3, randomized, double-blind, sham-procedure controlled study conducted in 121 symptomatic infants ≤7 months of age, diagnosed with SMA (symptom onset before 6 months of age). CS3B was designed to assess the effect of Spinraza on motor function and survival. Patients were randomized 2:1 to either Spinraza (as per the approved dosing regimen) or sham-control, with a length of treatment ranging from 6 to 442 days.
The median age of onset of clinical signs and symptoms of SMA was 6.5 weeks and 8 weeks for Spinraza treated versus sham-control patients respectively, with 99% of patients having 2 copies of the SMN2 gene and therefore deemed most likely to develop Type I SMA. The median age when patients received their first dose was 164.5 days for treated patients, and 205 days for sham-control. Baseline disease characteristics were largely similar in the Spinraza treated patients and sham-control patients except that Spinraza treated patients at baseline had a higher percentage compared to sham-control patients of paradoxical breathing (89% vs 66%), pneumonia or respiratory symptoms (35% vs 22%), swallowing or feeding difficulties (51% vs 29%) and requirement for respiratory support (26% vs 15%).
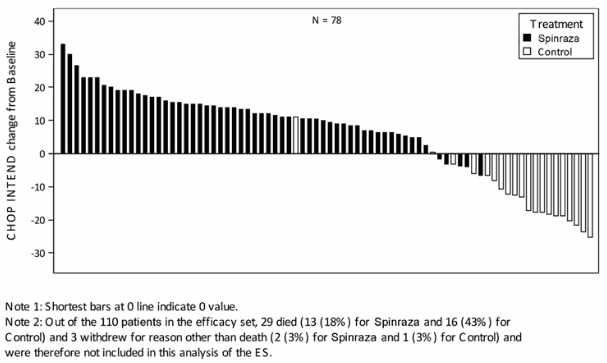
At the final analysis, a statistically significant greater percentage of patients achieved the definition of a motor milestone responder in the Spinraza group (51%) compared to the sham-control group (0%) (p<0.0001). Time to death or permanent ventilation (≥16 hours ventilation/day continuously for >21 days in the absence of an acute reversible event or tracheostomy) was assessed as the primary endpoint. Statistically significant effects on event-free survival, overall survival, the proportion of patients achieving the definition of a motor milestone responder, and the percentage of patients with at least a 4-point improvement from baseline in Children’s Hospital of Philadelphia Infant Test for Neuromuscular Disease (CHOP INTEND) score were observed in patients in the Spinraza group compared to those in the sham-control group (Table 3).
In the efficacy set, 18 patients (25%) in the Spinraza group and 12 patients (32%) in the sham-control group required permanent ventilation. Of these patients, 6 (33%) in the Spinraza group and 0 (0%) in the sham-control group met the protocol-defined criteria for a motor-milestone responder.
Table 3. Primary and secondary endpoints at final analysis – Study CS3B:
| Efficacy Parameter | Spinraza treated Patients | Sham-control Patients |
|---|---|---|
| Survival | ||
| Event-free survival2 | ||
| Number of patients who died or received permanent ventilation | 31 (39%) | 28 (68%) |
| Hazard ratio (95% CI) | 0.53 (0,.2–0.89) | |
| p-τιμή6 | p=0.0046 | |
| Overall survival2 | ||
| Number of patients who died | 13 (16%) | 16 (39%) |
| Hazard Ratio (95% CI) | 0.37 (0.18–0.77) | |
| p-τιμή6 | p=0.0041 | |
| Motor function | ||
| Motor milestones3 | ||
| Proportion achieving pre-defined motor milestone responder criteria (HINE section 2)4,5 | 37 (51%)1 p<0.0001 | 0 (0%) |
| Proportion at Day 183 | 41% | 5% |
| Proportion at Day 302 | 45% | 0% |
| Proportion at Day 394 | 54% | 0% |
| Proportion with improvement in total motor milestone score | 49 (67%) | 5 (14%) |
| Proportion with worsening in total motor milestone score | 1 (1%) | 8 (22%) |
| CHOP INTEND3 | ||
| Proportion achieving a 4-point improvement | 52 (71%) p<0.0001 | 1 (3%) |
| Proportion achieving a 4-point worsening | 2 (3%) | 17 (46%) |
| Proportion with any improvement | 53 (73%) | 1 (3%) |
| Proportion with any worsening | 5 (7%) | 18 (49%) |
1 CS3B was stopped following positive statistical analysis on the primary endpoint at interim analysis (statistically significantly greater percentage of patients achieved the definition of a motor milestone responder in the Spinraza group (41%) compared to the sham-control group (0%), p<0.0001)
2 At the final analysis, event-free survival and overall survival were assessed using the Intent to Treat population (ITT Spinraza n=80; Sham-control n=41).
3 At the final analysis, CHOP INTEND and motor milestone analyses were conducted using the Efficacy Set (Spinraza n=73; Sham-control n=37).
4 Assessed at the later of Day 183, Day 302, and Day 394 Study Visit
5 According to Hammersmith Infant Neurological Examination (HINE) section 2: ≥2 point increase [or maximal score] in ability to kick, OR ≥1 point increase in the motor milestones of head control, rolling, sitting, crawling, standing or walking, AND improvement in more categories of motor milestones than worsening, defined as a responder for this primary analysis.
6 Based on log-rank test stratified by disease duration
The extent of improvement in CHOP INTEND is shown in Figure 1 (change from baseline score for each subject).
Figure 1. Change in CHOP INTEND from Baseline to Later of Day 183, Day 302, and Day 394 Study Visit – Endear Study/CS3B (Efficacy Set, ES):
To allow for long term follow up of these patients, at the end of Study CS3B, 89 patients (Spinraza: n=65; sham-control: n=24) enrolled in Study CS11 (SHINE). Study CS11 is an open-label extension study for SMA patients who previously participated in the other Spinraza clinical studies. In patients randomised to Spinraza in Study CS3B and including the extension of treatment with Spinraza in Study CS11, patients received the medication for 6 to 3043 days (median 2443 days). In patients randomised to sham in Study CS3B and initiating Spinraza in Study CS11, patients received the medication for 65 to 2520 days (median 2090 days).
Improvements in motor function were observed among patients continuing Spinraza from Study CS3B, as well as those who initiated Spinraza in Study CS11 (Figure 3), with the greatest benefit observed in those with earlier treatment initiation. The majority of patients were alive at their last visit after initiating treatment with Spinraza in either Study CS3B or Study CS11.
Patients initiating Spinraza in Study CS3B were of median age 5.5 months (range 1.7 to 14.9 months). From Spinraza initiation and including extension of treatment in Study CS11, the median time to death or permanent ventilation was 1.4 years. At the end of Study CS11, 60 out of 81 patients (74%) were alive and 41 out of 81 patients (51%) were alive and had not met the Study CS11 definition of permanent ventilation. Mean HINE-2 total motor milestonescore increased by 5.3 (SD 4.6; n=52) and CHOP INTEND score increased by 18.4 (SD 14.7; n=38) points from initiation of Spinraza to follow up visit day 394 and 2198 respectively.
Patients randomised to sham in Study CS3B and initiating Spinraza in Study CS11 were of a median age of 17.8 months (range 10.1 to 23.0 months). Prior to Spinraza initiation 12 out of 24 patients (50%) had met the Study CS11 definition of permanent ventilation. The median time to death or permanent ventilation was 2.76 years after initiation of Spinraza in Study CS11. At the end of Study CS11, 19 out of 24 patients (79%) were alive and 6 out of 12 patients (50%) were alive without permanent ventilation. Improvement in mean total motor milestone score of 1.4 (SD 1.8; n=12) and CHOP INTEND score of 11.5 (SD 12.2, n=10) scores were observed from Study CS11 baseline to follow up visit day 394 or 2198 respectively.
These results are supported by an open-label Phase 2 study in symptomatic patients diagnosed with SMA (CS3A). Median age of onset of clinical signs and symptoms was 56 days and patients had either 2 SMN2 gene copies (n=17) or 3 SMN2 gene copies (n=2) (SMN2 gene copy number unknown for 1 patient). Patients in this study were deemed most likely to develop Type I SMA. The median age at first dose was 162 days.
The primary endpoint was the proportion of patients who improved in one or more categories in motor milestones (according to HINE section 2: ≥2 point increase [or maximal score] in ability to kick or voluntary grasp or ≥1 point increase in the motor milestones of head control, rolling, sitting, crawling, standing or walking). Twelve out of 20 patients (60%) in the study met the primary endpoint with improvement in mean motor milestone achievement over time. An improvement in mean CHOP INTEND score over time was observed from baseline to day 1072 (mean change 21.30). Overall, 11 out of 20 patients (55%) met the endpoint of an increase in total CHOP INTEND score of ≥4 points as of the last study visit. Of the 20 subjects enrolled, 11 (55%) were alive and free of permanent ventilation at the last visit. Four patients met the criteria for permanent ventilation and five patients died during the study.
Later onset
Study CS4 (CHERISH) was a Phase 3, randomised, double-blind, sham-procedure controlled study conducted in 126 symptomatic patients with later-onset SMA (symptom onset after 6 months of age). Patients were randomized 2:1 to either Spinraza (dosed with 3 loading doses and maintenance doses every 6 months) or sham-control, with a length of treatment ranging from 324 to 482 days. The median age at screening was 3 years, and the median age of onset of clinical signs and symptoms of SMA was 11 months. The majority of patients (88%) have 3 copies of the SMN2 gene (8% have 2 copies, 2% have 4 copies, and 2% have an unknown copy number). At baseline, patients had a mean Hammersmith Functional Motor Scale Expanded (HFMSE) score of 21.6, a mean revised upper limb module (RULM) of 19.1, all had achieved independent sitting, and no patients had achieved independent walking. Patients in this study were deemed most likely to develop Type II or III SMA. Baseline disease characteristics were generally similar with the exception of an imbalance in the proportion of patients who had ever achieved the ability to stand without support (13% of patients in the Spinraza group and 29% in sham-control) or walk with support (24% of patients in the Spinraza group and 33% in sham-control).
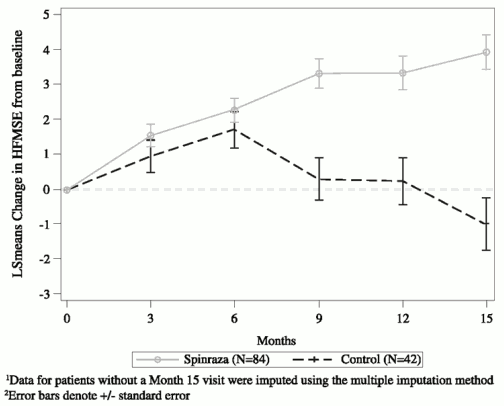
At the final analysis, a statistically significant improvement in HFMSE score from baseline to Month 15 was seen in the Spinraza group compared to the sham-control group (Table 3, Figure 2). The analysis was conducted in the ITT population (Spinraza: n=84; sham-control: n=42), and post-baseline HFMSE data for patients without a Month 15 visit were imputed using the multiple imputation method. An analysis of the subset of patients in the ITT population who had observed values at Month 15 demonstrated consistent, statistically significant results. Of those with observed values at Month 15, a higher proportion of Spinraza treated subjects had improvement (73% vs 41%, respectively) and a lower proportion of Spinraza treated subjects had worsening (23% vs 44%, respectively) in total HFMSE score compared to sham-control. Secondary endpoints including functional measures and WHO motor milestone achievement were formally statistically tested and are described in Table 4.
Initiation of treatment sooner after symptom onset resulted in earlier and greater improvement in motor function than those with delayed treatment initiation; however, both groups experienced benefit compared to sham control.
Table 4. Primary and secondary endpoints at final analysis – Study CS4 1:
| Spinraza treated Patients | Sham-control Patients | |
|---|---|---|
| HFMSE score | ||
| Change from baseline in total HFMSE score at 15 months1,2,3 | 3.9 (95% CI: 3.0, 4.9) p=0.0000001 | -1.0 (95% CI: -2.5, 0.5) |
| Proportion of patients who achieved at least a 3 point improvement from baseline to month 152 | 56.8% (95% CI: 45.6, 68.1) P=0.00065 | 26.3% (95% CI: 12.4, 40.2) |
| RULM | ||
| Mean change from baseline to month 15 in total RULM score2,3 | 4.2 (95% CI: 3.4, 5.0) p=0.00000016 | 0.5 (95% CI: -0.6, 1.6) |
| WHO motor milestones | ||
| Proportion of patients who achieved new motor milestones at 15 months4 | 19.7% (95% CI: 10.9, 31.3) p=0.0811 | 5.9% (95% CI: 0.7, 19.7) |
1 CS4 was stopped following positive statistical analysis on the primary endpoint at interim analysis (statistically significant improvement from baseline HFMSE score was observed in Spinraza treated patients compared to the sham-control patients (Spinraza vs. sham-control: 4.0 vs. -1.9; p=0.0000002))
2 Assessed using the Intent to Treat population (Spinraza n=84; Sham-control n=42); data for patients without a Month 15 visit were imputed using the multiple imputation method
3 Least squares mean
4 Assessed using the Month 15 Efficacy Set (Spinraza n=66; Sham control n=34); analyses are based on imputed data when there are missing data.
5 Based on logistic regression with treatment effect and adjustment for each subject’s age at screening and HFMSE score at baseline
6 Nominal p value
Figure 2. Mean change from baseline in HFMSE score over time at final analysis (ITT) – Study CS41,2:
Upon completion of Study CS4 (CHERISH), 125 (83 Spinraza and 42 sham) patients enrolled in Study CS11 (SHINE) where all patients received Spinraza. The majority of Spinraza treated patients experienced stabilization or improvement in motor function, with the greatest benefit observed in those with earlier treatment initiation.
Patients initiating Spinraza in Study CS4 were of a median age 4.1 years (range 2.1 to 9.2 years). From Spinraza initiation and including extension of treatment in Study CS11, patients received the medication for a median time of 7.2 years (range 1.3 to 8.4 years). HFMSE mean score increased 1.3 (SD 9.4 n=54) and RULM mean score increased by 6.4 (SD 6.5 n=54) at follow up visit day 2070.
Patients randomised to sham in Study CS4, initiated treatment with Spinraza in Study CS11 at a median age of 4.9 years (range 3.3 to 9.0 years). From Spinraza initation in Study CS11, patients received the medication for a median time of 5.8 years (range 2.7 to 6.7 years). HFMSE mean score decreased by 1.3 (SD 9.3 n=22) and RULM, score increased by 4.2 (SD 4.4 n=23) points at follow up visit day 2070.
In contrast, the natural disease course of untreated patients of similar age and clinical characteristics shows a progressive loss of motor function over time, with an estimated mean decline in HFMSE of 6.6 points over a similar period of 5 years.
These results are supported by 2 open label studies (study CS2 and study CS12). The analysis included 28 patients who received their first dose in study CS2, and then transferred to the extension phase, study CS12. The studies enrolled patients who were between 2 to 15 years of age at first dose. Of the 28 patients, 3 were at least 18 years of age at their last study visit. 1 out of 28 patients had 2 SMN2 gene copies, 21 had 3 copies, and 6 had 4 copies.
Patients were assessed over a 3 year treatment period. A sustained improvement was seen in patients with Type II SMA who experienced a mean improvement from baseline HFMSE score of 5.1 (SD 4.05, n=11) at Day 253, and 9.1(SD 6.61, n=9) at Day 1050. The mean total score was 26.4 (SD 11.91) at Day 253 and 31.3 (SD 13.02) at Day 1050, no plateau was observed. Patients with Type III SMA demonstrated a mean improvement from baseline HFMSE score of 1.3 (SD 1.87, n=16) at Day 253 and 1.2 (SD 4.64, n=11) at Day 1050. The mean total score was 49.8 (SD 12.46) at Day 253 and 52.6 (SD 12.78) at 1050 days.
In patients with Type II SMA the Upper Limb Module test was conducted with mean improvement of 1.9 (SD 2.68, n=11) at Day 253 and 3.5 (SD 3.32, n=9) at Day 1050. The mean total score was13.8 (SD 3.09) at Day 253 and 15.7 (SD 1.92) at Day 1050.
The 6MWT (six-minute walk test) was conducted for ambulatory patients only. In these patients, a mean improvement of 28.6 meters (SD 47.22, n=12) at Day 253 and 86.5 metres (SD 40.58, n=8) at Day 1050. The mean 6MWT distance was 278.5 meters (SD 206.46) at Day 253 and 333.6 metres (SD 176.47) at Day 1050. Two previously non-independent ambulatory patients (Type III) achieved independent walking, and one non-ambulatory patient (Type II) achieved independent walking.
An additional clinical study, CS7 (EMBRACE) was opened for patients not eligible for participation in Study CS3B or Study CS4 due to screening age or SMN2 copy number. CS7 is a phase 2, randomized, double-blind, sham-procedure study in symptomatic patients diagnosed with infantile-onset SMA (≤6 months) or later-onset SMA (>6 months) and 2 or 3 copies of SMN2 (Part 1), followed by a long-term open-label extension phase (Part 2). In Part 1 of the study, patients were followed for a median of 302 days.
All patients who received Spinraza were alive as of the early termination of Part 1, however, one patient in the control arm died at Study Day 289. In addition, no patients in the Spinraza or sham- control group required the use of permanent ventilation. Of the 13 patients with infantile-onset SMA, 7 of out 9 patients (78%; 95%CI: 45, 94) in the Spinraza group and 0 out of 4 patients (0%; 95%CI: 0, 60) in the sham group met the criteria for motor milestone response (according to HINE section 2: ≥2 point increase [or maximal score] in ability to kick OR ≥1 point increase in the motor milestones of head control, rolling, sitting, crawling, standing or walking and improvement in more categories of motor milestones than worsening). Of the 8 patients with later-onset SMA, 4 out of 5 patients (80%; 95% CI: 38, 96) in the Spinraza group and 2 out of 3 (67%; 95% CI: 21, 94) in the sham-control group met this definition of response.
Adult
Real world clinical findings support the effectiveness of nusinersen to stabilize or improve motor function in some SMA adult Type II and III patients.
By month 14 of nusinersen treatment, the number of patients with a clinically meaningful improvement from baseline on HFMSE (≥ 3 points) was 53 out of 129 patients, the number of patients with clinically meaningful improvement on the RULM (≥ 2 points) was 28 out of 70 and among walkers 25 out of 49 for the 6MWT (≥ 30 meters).
The safety data in the adult population are consistent with the known safety profile of nusinersen and with co-morbidities associated with the underlying disease of SMA.
Presymptomatic infants
Study CS5 (NURTURE) is an open-label study in presymptomatic infants genetically diagnosed with SMA, who were enrolled at 6 weeks of age or younger. Patients in this study were deemed most likely to develop Type I or II SMA. Median age at first dose was 22 days.
An interim analysis was conducted when patients had been on study for a median of 48.3 months (36.6 to 57.1 months) and were of a median age at last visit of 46.0 months (34.0 to 57.1 months). At the interim analysis, all 25 patients (2 SMN2 gene copies, n=15; 3 SMN2 gene copies, n=10) were alive without permanent ventilation. The primary endpoint, time to death or respiratory intervention (defined as invasive or non-invasive ventilation for ≥6 hours/day continuously for ≥7 consecutive days or tracheostomy), could not be estimated as there were too few events. Four patients (2 SMN2 copies) required respiratory intervention >6 hours/day continuously for ≥7 days, all of whom initiated ventilatory support during an acute reversible illness.
Patients achieved milestones unexpected in Type I or II SMA and more consistent with normal development. At the interim analysis, all 25 (100%) patients had achieved the WHO motor milestone of sitting without support, 23 (92%) patients were walking with assistance and 22 (88%) had achieved walking alone. Twentyone (84%) patients achieved the maximum attainable CHOP INTEND score of 64. All patients had the ability to suck and swallow at last visit (Day 788), with 22 (88%) infants achieving a maximal score on the HINE Section 1.
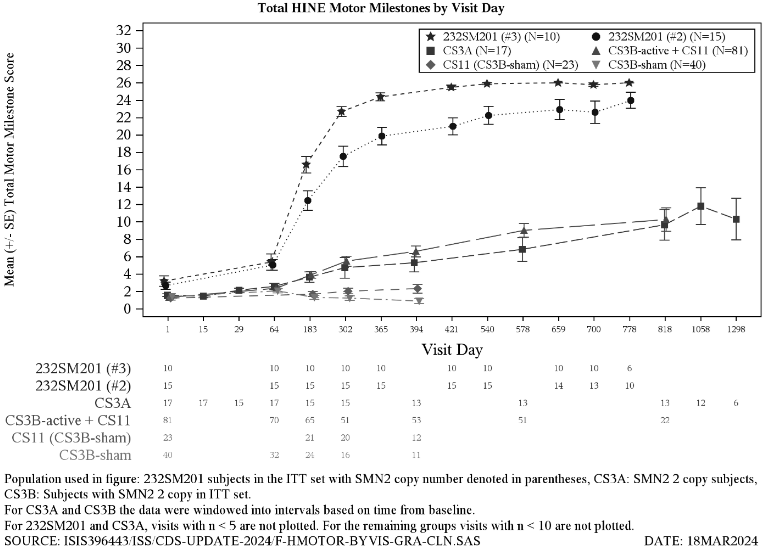
Patients developing clinically manifested SMA was assessed at Day 700 visit. The protocol-defined criteria for clinically manifested SMA included age-adjusted weight below the fifth WHO percentile, a decrease of 2 or more major weight growth curve percentiles, the placement of a percutaneous gastric tube, and/or the inability to achieve expected age-appropriate WHO milestones (sitting without support, standing with assistance, hands-and-knees crawling, walking with assistance, standing alone and walking alone). At day 700, 7 out of 15 patients (47%) with 2 SMN2 gene copies and 0 out of 5 patients (0%) with 3 SMN2 copies, met the protocol-defined criteria of clinically manifested SMA, however, these patients were gaining weight and achieving WHO milestones, inconsistent with Type I SMA. A comparison of motor milestone achievement among the patients with symptomatic infantile-onset SMA and presymptomatic SMA is shown in Figure 3.
Figure 3. Change in HINE Motor Milestones versus Study days for Study CS3B (treated and sham-control), CS3A, CS5 and CS11:
Pharmacokinetic properties
Single- and multiple-dose pharmacokinetics (PK) of nusinersen, administered via intrathecal injection, were determined in paediatric patients diagnosed with SMA.
Absorption
Intrathecal injection of nusinersen into the CSF allows nusinersen to be fully available for distribution from the CSF to the target central nervous system (CNS) tissues. The average increase in trough CSF levels from the start of the maintenance phase through to the last observation timepoint across all patients was approximately 3.2-fold and 2.3-fold in the later-onset and infantile-onset populations respectively. Overall, cumulative CSF PK data collected through to the end of CS11 indicated that in infantile and later onset SMA patients, the standard dosing regimen (12 mg every 4 months) leads to a steady state CSF concentration by 7 to 8 years of treatment. Following intrathecal administration trough plasma concentrations of nusinersen were relatively low compared to the trough CSF concentration. Median plasma Tmax values ranged from 1.7 to 6.0 hours. Mean plasma Cmax and AUC values increased approximately dose proportionally over the evaluated dose range. There is no accumulation in plasma exposure measures (Cmax and AUC) after multiple doses.
Distribution
Autopsy data from patients (n=3) show that nusinersen administered intrathecally is broadly distributed within the CNS achieving therapeutic levels in the target spinal cord tissues. Presence of nusinersen was also demonstrated in neurons and other cell types in the spinal cord and brain, and peripheral tissues such as skeletal muscle, liver, and kidney.
Biotransformation
Nusinersen is metabolized slowly and predominantly via exonuclease (3'- and 5')-mediated hydrolysis and is not a substrate for, or inhibitor or inducer of CYP450 enzymes.
Elimination
The mean terminal elimination half-life in CSF is estimated at 135 to 177 days. The primary route of elimination is expected via urinary excretion of nusinersen and its metabolites.
Interactions
In vitro studies indicated that nusinersen is not an inducer or inhibitor of CYP450-mediated oxidative metabolism and therefore should not interfere with other medicinal products for these metabolic pathways. Nusinersen is not a substrate or inhibitor of human BCRP, P-gp, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, or BSEP transporters.
Characteristics in specific patient populations
Renal and hepatic impairment
The pharmacokinetics of nusinersen in patients with renal or hepatic impairment has not been studied. The effect of hepatic or renal insufficiency as covariates could not be thoroughly evaluated in the population PK model given the rarity of patients displaying clinically relevant liver or kidney insufficiencies. Population PK analyses revealed no apparent correlation between hepatic and renal clinical chemistry markers and inter-subject variability.
Race
The majority of patients studied were Caucasian. The population PK analysis suggests that race is unlikely to affect the PK of nusinersen.
Preclinical safety data
Genotoxicity/Carcinogenity
Nusinersen demonstrated no evidence of genotoxicity. Nusinersen was not carcinogenic in a 2-year study in mice at plasma exposure levels 104-fold higher than in patients receiving 12 mg of maintenance nusinersen
Reproductive toxicity
Reproductive toxicology studies were conducted using subcutaneous administration of nusinersen in mice and rabbits. No impact on male or female fertility, or embryo-foetal development, or pre/post-natal development was observed.
Toxicology
In repeat-dose toxicity studies (14-weeks and 53-weeks) of intrathecal administration to juvenile cynomolgus monkeys, nusinersen was well tolerated. The exception was an acute, transient deficit in lower spinal reflexes which occurred at the highest dose levels in each study (3 or 4 mg per dose; equivalent to 30 or 40 mg per intrathecal dose in patients). These effects were observed within several hours post-dose and generally resolved within 48 hours.
In the 53-week intrathecal dosing study in cynomolgus monkeys no toxicity effects were seen at levels up to 14-fold the recommended annual clinical maintenance dose.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.