SULFAMYLON Powder for solution Ref.[10361] Active ingredients: Mafenide
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
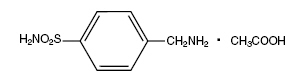
Mafenide acetate, USP is a synthetic antimicrobial agent designated chemically as α-amino-p-toluenesulfonamide monoacetate.
It has the following structural formula:
Mafenide acetate, USP is a white, crystalline powder which is freely soluble in water.
SULFAMYLON for 5% Topical Solution is provided in packets containing 50 g of sterile mafenide acetate to be reconstituted in 1000 mL of Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. After mixing, the solution contains 5% w/v of mafenide acetate. The solution is an antimicrobial preparation suitable for topical administration. The solution is not for injection. The reconstituted solution may be held up to 28 days after preparation if stored in unopened containers. ONCE A CONTAINER IS OPENED, ANY UNUSED PORTION SHOULD BE DISCARDED AFTER 48 HOURS. Store the reconstituted solution at 20° to 25°C (68° to 77°F). Limited storage periods at 15° to 30°C (59° to 86°F) are acceptable.
| How Supplied |
|---|
|
SULFAMYLON (mafenide acetate, USP) for 5% Topical Solution is available in packets (NDC 51079-624-84) containing 50 g of sterile mafenide acetate to be prepared using 1000 mL Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. (See DOSAGE AND ADMINISTRATION: SULFAMYLON for 5% Topical Solution: Directions for Preparation of the Solution.) The packets are supplied as follows: Carton of five 50 g packets – NDC 51079-624-85 Mylan Institutional Inc.: Rockford, IL 61103 U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| SULFAMYLON | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.